Introduction
Biofilm formation is one of the most important factors influencing wound infections and may result in biofilm-related sepsis, which is the major cause of wound-related mortality. Organized bacterial biofilm communities enhance antibiotic resistance, complicate the treatment and result in development of chronic infection [1–4]. Literature data indicate that approximately three-fourths of deaths among burned patients occur due to wound bacterial infections, mostly with Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa [1, 5]. These bacteria are able to produce biofilm, which protects them against the host immune system as well as antibiotic therapy.
Staphylococcus aureus is frequently isolated from patients with therapeutically challenging wound infections [6]. Moreover, infections with methicillin-resistant S. aureus strains (MRSA) which additionally produce biofilm are recognized as extremely hard to medicate. Stress conditions such as antibiotics, sodium chloride, temperature, and oxidative stress can induce biofilm formation by S. aureus [7]. Yin et al. suggest that serum of a burned patient increases the biofilm formation by S. aureus via the induction of oxidative stress [8].
In recent decades there has been growing interest in activity of plant metabolites. This is due to the development of disciplines such as biochemistry, phytochemistry, pharmacology and genetics. One of the biggest plant families on earth is named the Apiaceae family and represents many valuable biological activities. According to available data, plants such as Bupleurum falcatum L., Falcoria vulgaris Bernh., Ferula gummosa Boiss., Ferulago angulata (Schltdl.) Boiss. and Ferulago carduchorum Boiss. & Hausskn. ex Boiss are used in the treatment of wound infections [9]. There is also increasing interest in different strategies used to prevent biofilm formation. Essential oils have been considered an effective substitute for commonly used antibiotics as well as an addition to conventional therapy [10, 11]. Simultaneously, in recent times it has been observed that conventional chemical drugs can generate many harmful side effects but also bacteria themselves are able to produce a number of resistance mechanisms. This problem is especially important due to the increase in the quantity of nosocomial infections.
Foeniculum vulgare Mill. (fennel) from the Apiaceae family is widely cultivated worldwide [12]. Fennel is a very interesting and safe aromatic herb with medicinal applications. Fennel essential oil (FEO) has many beneficial medical properties, including antibacterial, antifungal, anti-inflammatory, and antioxidant effects [13].
Burn-wounded patients are mostly managed in highly profiled intensive care units. Such management involves a number of invasive procedures required for constant monitoring, drug administration, etc. These involve artificial materials, for which the issue of occurrence of bacterial biofilm arises. Moreover, as aseptic procedures represent an extremely significant factor in successful recovery from burns, a great spectrum of antiseptic measurements is being applied daily in burn intensive care units.
Aim
The aim of the study was to evaluate the synergistic effect of FEO and H2O2 on biofilm formation by S. aureus reference strains.
Material and methods
Strains and culture conditions
Three reference strains were used in this study: S. aureus ATCC 29213 (methicillin-sensitive S. aureus – MSSA), S. aureus ATCC 43300 (MRSA) and S. aureus ATCC 6538 (MSSA) as a reference biofilm forming strain [14]. The bacteria were cultivated for 18 h at 37°C on Columbia agar with 5% sheep blood (bioMérieux, Warsaw, Poland).
Essential oil analysis
FEO used in the study was purchased from Pollena-Aroma (Warsaw, Poland). FEO composition was analysed by gas chromatography with mass spectrometry (GC-MS) at the Faculty of Chemical Technology and Engineering, West Pomeranian University of Technology in Szczecin [15].
Determination of minimum inhibitory concentration
Minimum inhibitory concentration (MIC) values were determined separately for FEO and H2O2 (Chempur, Piekary Œl¹skie, Poland) against S. aureus reference strains by the broth microdilution method according to Clinical and Laboratory Standards Institute [16], but with our own modification: a final concentration of 1% (v/v) autoclaved Tween 80 (Sigma-Aldrich, Darmstadt, Germany) was incorporated into the medium to enhance FEO solubility. Two-fold dilutions (250 µl/ml – 0.12 µl/ml for FEO, and 40 – 0.04 mM for H2O2) were performed; each well containing 50 µl of tested chemicals and 50 µl of bacterial suspension at the final concentration of 106 CFU/ml. All tests were performed in triplicate. The MIC was estimated after 18 h of incubation at 35°C in Mueller-Hinton Broth (Sigma-Aldrich, Darmstadt, Germany) using resazurin (Sigma-Aldrich, Darmstadt, Germany) [17]. In order to exclude an inhibitory effect of 1% Tween 80 on the S. aureus growth, control assays with MHB and MHB supplemented with 1% Tween 80 were performed. Using the known density of FEO, the final result was expressed in mg/ml.
Checkerboard assay
The checkerboard method was performed according to Yap et al. [18] with the following modifications: the final concentration of bacterial suspension was 106 CFU/ml and resazurin was added to each plate well after 18 h of incubation. To determine MICs for individual mixtures of both compounds, serial dilutions of FEO (250 µl/ml – 0.12 µl/ml) and H2O2 (40 – 0.04 mM) were combined in 96-well polystyrene microtiter plates. Each well contained: 25 µl of the appropriate concentration of FEO, 25 µl of the appropriate concentration of H2O2 and 50 µl of bacterial suspension containing a final concentration of 106 CFU/ml in each well. The plates were incubated at 35°C for 18 h. All determinations were performed in duplicate. The MICs of both FEO and H2O2 in combination and alone were determined as described above. The combined effects of the FEO and H2O2 were calculated and expressed in terms of a fractional inhibitory concentration index (FICI) using the following formula: FIC = MIC of FEO or H2O2 in combination/MIC of FEO or H2O2, FICI = FIC of FEO + FIC of H2O2. Results were interpreted as synergy (FICI ≤ 0.5), no interaction (FICI > 0.5–4.0) or antagonism (FICI > 4.0) [18].
Biofilm formation and assessment of essential oil biofilm formation prevention
Biofilm formation assay was performed according to Latimer et al. [19] with minor modifications. Overnight cultures of S. aureus ATCC 6538 were diluted 1 : 200 with lysogeny broth (LB) medium (Sigma-Aldrich, Darmstadt, Germany) and aliquots of 200 µl were transferred to 96-well polystyrene microtiter plates and incubated stationary at 37°C for 24 h. In order to evaluate the prevention of biofilm formation, FEO was added in 0.5 MIC and 0.25 MIC concentrations. Determination of the H2O2 effect on biofilm formation was performed by the addition of 0.5 MIC of H2O2 to a cell suspension which contained 0.5 and 0.25 MIC concentration of FEO. After incubation, wells were emptied and washed five times with PBS to remove planktonic and unattached cells.
The cell viability and biofilm mass were measured in the resazurin assay and crystal violet staining according to Skogman et al. [20] with minor modifications. FEO treated wells containing S. aureus biofilm were filled with 200 µl of fresh LB containing 20 mM of resazurin (Sigma-Aldrich, Darmstadt, Germany) and incubated for 20 min at 37°C with shaking (200 rpm). After the incubation, medium was removed and fluorescence (λex = 520 nm; λem = 590 nm) was measured. Wells were washed three times using PBS buffer (Sigma-Aldrich, Darmstadt, Germany) for the biomass quantification. At this stage,200 µl of methanol (Sigma-Aldrich, Darmstadt, Germany) was added to each well for fixation and incubated for 20 min. Afterwards, methanol was removed, and plates were air dried. Staining was performed with 200 µl of crystal violet (1% w/v, Sigma-Aldrich, Darmstadt, Germany) added to wells and further incubated for 20 min at room temperature. Pigment was rinsed with sterile water. 200 µl of ethanol: acetone (8 : 2, v/v) solution was added to each well and biofilm was quantified by determining the optical density at 590 nm.
The influence of FEO and combination of FEO with H2O2 on S. aureus biofilm formation was calculated asa % of formed biofilm compared to the control. The control for the influence of FEO was a biofilm formation assay with Tween 80. For combination of FEO and hydrogen peroxide the 0.5 MIC of H2O2 with Tween 80 was used as a control.
Results
Chemical analysis of FEO
The GC-MS analysis enabled identification of eleven compounds. The main constituent of FEO was trans-anethole (77.9%) followed by fenchone (12.8%), α-pinene (3.8%), estragole (2.3%) and limonene (2.1%) [15].
Antistaphylococcal inhibitory activity of FEO and H2O2
The results revealed that all S. aureus reference strains were susceptible to FEO and H2O2. The highest activity of FEO and H2O2 was determined. The combinations of FEO and H2O2 showed synergistic effects (FICI = 0.43–0.5) on all S. aureus reference strains. It was observed that the most effective combination of FEO and H2O2 decreased the MIC of FEO from 75.9 ±21.5 mg/ml to 0.12 mg/ml. Moreover, it was also noted that the addition of 1% Tween 80 had no impact on the growth of reference strains. The results of MICs and checkerboard assay against S. aureus reference strains are summarized in Table 1.
Table 1
Minimal inhibitory concentration (MIC), fractional inhibitory concentration (FIC) and FIC indices of fennel essential oil–hydrogen peroxide (FEO–H2O2) pairs against S. aureus reference strains
Biofilm formation
Results presented in Figure 1 A showed that subinhibitory concentrations (0.5 MIC and 0.25 MIC) of FEO significantly decreased the production of biofilm biomass in S. aureus strains and reduced the metabolic activity of attached cells. Statistically significant (p < 0.001) differences were observed for all analysed strains when the FEO-treated group was compared to the control. Moreover, the addition of H2O2 (Figure 1 B) resulted in statistically significant reduction of biofilm biomass compared to the control (p < 0.001) and 0.5 MIC FEO-only treated cells (p < 0.05).
Figure 1
Effect of subinhibitory concentration of fennel essential oil (FEO) (A) and subinhibitory concentration of FEO in combination with 0.5 MIC of H2O2 (B) on process of biofilm biomass and metabolic activity formation
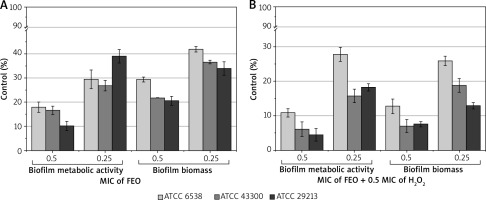
The results also showed that used 1% Tween 80 concentration decreased the level of S. aureus biofilm formation to 89.56% compared to the control without Tween 80. Additionally, the concentration of H2O2 used increased the biofilm formation level to 116.78% compared to the control.
Discussion
Infections represent the major challenge in the management of wounds. In clinical practice, burn wounds are particularly difficult to heal. Furthermore, due to the protein imbalance accompanying a severe burn-related condition, burn wounded patients are recognized as extremely highly susceptible to systemic infections and require specialized intensive care. Therefore, antimicrobial agents represent a significant tool in the intensive care of burn wounds, which additionally act as an infection gate. Hence there is an emerging need for safe and efficient antiseptics dedicated to topical application at different stages of wound healing. Topical antiseptics appear more beneficial compared to systemic antibiotics as they do not generate the emergence of multidrug resistance, represent a broader antimicrobial spectrum and additionally are less likely to provoke allergic reactions. Several well-known basic ones are used to decontaminate the wounds, including H2O2, acetic acid, iodine, chlorhexidine, silver sulfadiazine and silver nitrate. A number of studies have shown both their advantages and disadvantages, but the majority of clinical trials confirmed their safety and lack of a negative influence on wound healing [21]. Recently, many scientific centres have conducted research on synergistic or additive action of antiseptic agents. Current literature presents data on an increased activity of H2O2 combined with chlorhexidine against Streptococcus sobrinus, Enterococcus faecalis and S. aureus in oral infections [22, 23]. H2O2 also presents synergistic as well as additive activity with iodine against other Gram-positive and Gram-negative bacteria and yeast [24]. Other authors reported that H2O2 in combination with alcohols at concentrations of 3% and 5% rapidly eradicates S. epidermidis biofilms from surfaces of implant materials [25]. Grønseth et al. showed also the antibacterial efficacy of Lugol’s solution, acetic acid, and boric acid against S. aureus biofilm [26]. They found high effectiveness of Lugol’s solution against biofilm consisting of S. aureus clinical strains. Another study confirmed the anti-biofilm potential of essential oils and their major components as well [27]. H2O2 is still a commonly used and cheap agent for wound decontamination. Regarding this we decided to investigate the possible prevention of S. aureus biofilm formation by H2O2 combined with FEO. The GC-MS analysis of FEO showed that its composition was consistent with the European Pharmacopoeia recommendations [28]. Our previous study showed that the FEO possesses strong antistaphylococcal activity and increases the antimicrobial activity of cefoxitin, mupirocin, co-trimoxazole and ciprofloxacin [15]. Our current study found that the combination of FEO and H2O2 reveals a synergistic effect against reference strains: S. aureus ATCC 6538, S. aureus ATCC 43300 and S. aureus ATCC 29213. Furthermore, we proved that subinhibitory concentrations 0.5 and 0.25 MIC of FEO successfully decrease the production of biofilm biomass and reduce the metabolic activity of attached cells. Moreover, our studies confirmed that 1% Tween 80 concentration decreased the level of S. aureus biofilm formation, in contrast to H2O2, which increased its formation. These results correspond with published data of Toutain-Kidd et al. and Kulkarni et al. [29, 30]. It is known that the etiological factors of wound infections are not only bacterial pathogens, but also fungi such as yeast and dermatophytes. Essential oils of plants from the Apiaceae family also have antifungal activity inter alia against Candida spp., Trichophyton spp. and Microsporum spp. [31].
Conclusions
Our studies indicated that FEO containing mainly trans-anethole have a great potential for biofilm prevention. Moreover, these results will encourage for further research in order to determine the balanced level of activity dependently on the exposure time and anti-biofilm mechanism of FEO with H2O2. Because of the low toxicity of fennel essential oil and also the main constituent, trans-anethole, they can be a valuable addition to preparations used in challenging wound infections caused by MSSA and MRSA isolates.








