Introduction
CD163 belongs to group B of the scavenger receptor cysteine-rich (SRCR) superfamily [1]. It is exclusively expressed by mononuclear phagocytes as a type I transmembrane protein which synthesis is regulated by anti- and pro-inflammatory signals [2]. Moreover, CD163 is also detected as a soluble protein in body fluids including plasma [3]. The main mechanism responsible for appearance of soluble CD163 in body fluids is thought to be shedding of CD163 from the cell surface of the mononuclear phagocytes [4]. At least two enzymes have been implicated in this process: matrix metalloproteinase-9 (MMP-9) [5] and tumour necrosis factor α converting enzyme (TACE/ADAM17) [6]. Increased activity of MMP-9 has been associated with elevated levels of sCD163 in body fluids [7].
Expression of CD163 is strongly upregulated by anti-inflammatory mediators including corticosteroids (CS) and interleukin-10 (IL-10) [8]. Corticosteroids are very effective inducers of CD163 expression and the magnitude of upregulation of CD163 expression depends on the potency of the CS [9]. Those having the greatest affinity for the CS receptor are the most potent for upregulating CD163 expression [10]. Simultaneous application of dexamethasone and IL-10 to macrophages cultured in vitro exerts an additive effect on CD163 expression [11]. Some studies demonstrated anti-inflammatory effects of CD163 [12–14]. Soluble CD163 inhibits in a dose-dependent manner phorbol ester-induced T cell proliferation in vitro [13]. This anti-inflammatory function of CD163 seems to be restricted to its soluble form as membrane-bound CD163 does not exert such an effect [14]. The associations between T cell proliferation and CD163 expression come also from in vivo studies in which expression of CD163 was inversely correlated with markers of T cell proliferation [15]. Macrophages from lymphoid follicles, which are located in a place where intensive lymphocyte proliferation occurs, express little or no CD163 at all [16]. Moreover, sCD163 modulates cytokine release induced by house dust mite allergens by peripheral blood mononuclear cells in vitro leading to augmented IL-10 secretion [17].
Our previous studies demonstrated that circulating monocytes of asthmatic patients, in particular those with severe asthma, have a greater expression of CD163 than those of healthy subjects [18, 19]. Moreover, systemic corticosteroid therapy leads to significant upregulation of CD163 expression on circulating monocytes [19]. Recently, the elevated serum sCD163 level has been linked to overweight/obesity and increased risk of asthma exacerbation in pregnant asthmatic women [20]. Therapy of mild-moderate asthmatic patients with inhaled corticosteroids leads to a dramatic increase in sCD163 concentration in induced sputum indicating a strong effect of corticosteroids on sCD163 production locally at the site of inflammatory response [21]. However, little is known on the effect of systemic corticosteroid therapy on CD163 production in asthmatic patients.
Aim
The aim of the current study was to evaluate the serum concentration of sCD163 and ex vivo release of this protein by peripheral blood mononuclear cells in patients with and without systemic corticosteroid therapy.
Material and methods
The study was performed in 35 allergic asthma (AAs) patients including 15 treated with inhaled corticosteroids (ICS), 10 treated with oral corticosteroids (OCS) and 10 during asthma exacerbation (EX) before OCS had been started. In addition, 13 non-atopic healthy subjects were included as a control group. After asthma diagnosis its severity was assessed according to the Global Initiative for Asthma (GINA) criteria. In all patients, forced expiratory volume within the 1st s (FEV1) of less than 80% of the predicted value with at least 12% improvement 15 min after inhalation of 400 μg salbutamol was demonstrated. In OCS-AAs, a stable dose of oral corticosteroids had been used for at least 14 days before the study. Evaluation of EX-AAs was performed before systemic corticosteroid therapy was introduced. Asthma exacerbation was defined as progressive deterioration of lung function, which ultimately required therapy with systemic corticosteroids. Patients with any other systemic diseases or smoking history were not included in the study.
Venous blood was collected between 7 and 9 A.M. with heparin as an anticoagulant for cell isolation and without an anticoagulant for serum preparation. The study was approved by the local Ethics Committee. All participants provided written informed consent.
Cell isolation and culture
Peripheral blood mononuclear cells were isolated from 20 ml of heparin-anticoagulated venous blood by centrifugation with the use of Histopaque (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s protocol. The total number and viability of isolated cells were assessed using Fuchs Rosenthal chamber and trypan blue exclusion method. The cells were cultured in 24-well culture plates at a density of 1 × 106 cells/ml in RPMI-1640 medium (Sigma-Aldrich) supplemented with 5% heat inactivated foetal calf serum (Sigma-Aldrich), 25 mM L-glutamine (Sigma-Aldrich), 1% penicillin/streptomycin solution (Sigma-Aldrich).
After 24 (T24) and 144 (T144) h the supernatants were separated from the cells, aliquoted and stored frozen at –80°C until tested. In 6 HCs the supernatants were collected after 12, 24, 48, 72 and 144 h of culture to evaluate a time-dependent effect on release of sCD163 ex vivo.
Results
There was no significant difference of demographic parameters between the studied groups (Table 1). The asthmatic patients (n = 35) had lower mean FEV1 (70.6 ±19.3% predicted) than healthy subjects (HCs) (104.9 ±11.6% predicted; p < 0.001). Among asthmatics the greatest FEV1 was demonstrated in ICS-AAs (78.5 ±22.2% predicted) which was significantly less than in HCs (p < 0.001) but greater than that in EX-AAs (p = 0.008). The daily dose of ICS differed significantly in individual subgroups of asthmatic patients. The greatest daily dose of ICS was received by EX-AAs (1400 ±283 μg), significantly greater than that in OCS-AAs (1080 ±329 μg; p = 0.032) and in ICS-AAs (560 ±241 μg; p < 0.001).
Table 1
Patients’ characteristics
| Parameter | HCs (n = 13) | ICS-AAs (n = 15) | OCS-AAs (n = 10) | EX-AAs (n = 10) | P-value |
|---|---|---|---|---|---|
| Age [years] | 30.9 ±10.7 | 34.8 ±11.6 | 37.8 ±13.4 | 33.8 ±14.6 | 0.612 |
| Sex (female/male) | 6/7 | 6/9 | 4/6 | 5/5 | 0.95 |
| FEV1 (% predicted) | 105±11.4 | 78.5 ±22.2* | 72.1 ±16.4* | 57.1 ±7.7*^# | < 0.01 |
| BMI | 23.8 ±2.7 | 24.6 ±3.8 | 25.6 ±4.7 | 26.1 ±3.9 | 0.484 |
| ICS [μg/day] | – | 560 ±241 | 1080 ±329 | 1400 ±283 | – |
| OCS [mg/day] | – | – | 24.5 ±10.7 | – | – |
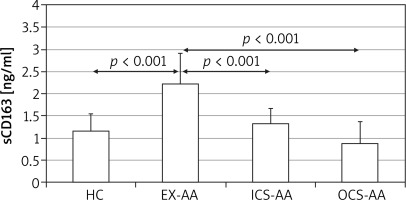
There was no significant difference among serum sCD163 concentrations between HCs, ICS-AAs and OCS-AAs (Figure 1). However, EX-AAs were characterized by a significantly greater serum sCD163 concentration in comparison to all other subgroups (p < 0.001). No significant association between the serum sCD163 concentration and age, sex or body mass index (BMI) of the studied patients could be demonstrated (not shown).
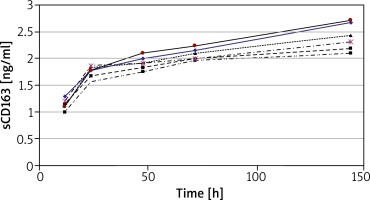
Analysis of sCD163 production in relation to time of cell culture was performed using peripheral blood mononuclear cells (PBMC) from 6 HCs. The concentration of sCD163 was evaluated in supernatants of cultures incubated for 12, 24, 48, 72 and 144 h (Figure 2). The concentration of sCD163 was already detected at T12 (mean 1.2 ±0.1 ng/ml) and increased with time in culture reaching the greatest concentration at T144 (mean 2.4 ±0.25 ng/ml) (Figure 2). For further studies only cultures of 24 or 144 h duration were used.
Figure 2
Concentration of sCD163 in supernatants of PBMC cultured for 12 to 144 h. The results present data obtained from 6 HCs
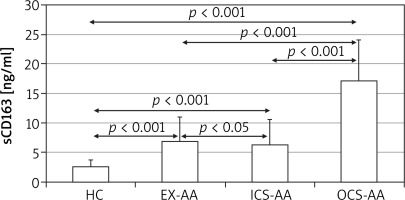
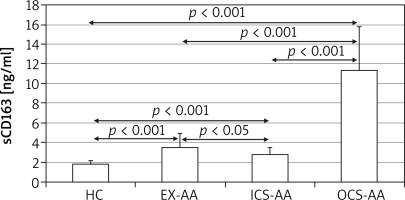
At T24 the mean concentration of sCD163 was greater in cell cultures of AAs (5.4 ±4.5 ng/ml) in comparison with HCs (1.8 ±0.35 ng/ml; p = 0.006) (Figure 3). The greatest concentration was demonstrated in OCS-AAs (11.3 ±4.52 ng/ml) which was significantly greater than that in ICS-AAs (2.77 ±0.7 ng/ml; p < 0.001) and in EX-AAs (3.52 ±1.44 ng/ml; p < 0.001). The mean sCD163 concentration at T24 was greater in each of the AAs subgroups than in HCs (p < 0.001 for all comparisons). Similar relations were demonstrated at T144, the greatest mean concentration of sCD163 was observed in OCS-AAs (17.1 ±7.0 ng/ml) which was significantly greater than in ICS-AAs (6.2 ±4.4 ng/ml; p < 0.001), EX-AAs (6.94 ±4.1 ng/ml; p < 0.001) and in HCs (2.52 ±1.17 ng/ml; p < 0.001) (Figure 4).
Discussion
To the best of our knowledge, this is the first study which demonstrates the differences in in vivo and ex vivo sCD163 production in asthmatic patients depending on their clinical status.
CD163 is reported to demonstrate an anti-inflammatory function at least two ways. First, it can stimulate intracellular signalling leading to secretion of various anti-inflammatory cytokines and heme metabolites, which is triggered after CD163 mediated delivery of haemoglobin to the macrophage [12]. Second, sCD163 inhibits inflammatory response influencing T cell proliferation [13, 14]. The expression of CD163 is induced by corticosteroids [22] and anti-inflammatory cytokines such as IL-10 and IL-6 [23]. On the other hand, secreted by Th2 type cells, IL-4 and IL-13 have been shown to downregulate the expression of CD163 influencing de novo CD163 synthesis [1]. A local expression of CD163 in the lungs during respiratory infection implies its role as an pulmonary defence element [24]. A reduced expression of CD163 on broncho-alveolar macrophages in patients with asthma suggests that its anti-inflammatory role in regulation of inflammatory responses in the lung of asthmatic patients may be impaired [25]. However, the reverse association of the serum sCD163 level with FEV1 in patients in asthma was observed [25]. This implicates that increased CD163 shedding already within circulation is associated with the loss of lung function. A mouse model of allergic asthma provided further evidence for an important role of CD163 in regulation of inflammatory response in the airways [25]. Genetically modified mice, which do not express CD163, were characterized by a greater airway inflammation in response to a house dust mite allergen challenge [26]. The beneficial effect of CD163 was dependent on its direct binding to a major house dust mite allergen Der p 1, which led to attenuated production of CCL24 [26]. Exogenous sCD163 enhance Dermatophagoides pteronyssinus allergen extract induced IL-10 release by peripheral blood mononuclear cells [17].
Our study is consistent with previously published studies which demonstrate the increase in the serum sCD163 concentration in chronic inflammatory diseases such as asthma and rheumatoid arthritis [26, 27]. In addition, it demonstrates that inflammation associated with asthma exacerbations leads to a systemic increase in sCD163, which is consistent with previous reports in pneumonia or chronic inflammatory diseases [28]. An increased activity of the enzymes participating in CD163 shedding in serum has been reported during asthma exacerbations [29]. In addition, altered fat metabolism significantly alters CD163 expression [27]. In some populations of obese patients, including obese asthmatic girls, an elevated serum sCD163 level was demonstrated [27]. However, in our patients we were not able to demonstrate any association between BMI or sex and sCD163 concentration, possibly due to a relatively homogenous population of our patients in terms of BMI and lack of truly obese patients included in our study. It seems that in our patients’ inflammation was the dominant factor affecting CD163 expression and its serum concentration.
For the first time we have demonstrated in asthma that systemic corticosteroid therapy, which leads to the remission of the asthma exacerbation, is not associated with a significant increase in serum sCD163 concentration. This occurs despite the fact that corticosteroids are potent inducers of CD163 expression in monocytes/macrophages [8]. However, peripheral blood monocytes derived from patients treated with OCS are characterized by a strong production of sCD163 ex vivo. This in part can be related to the enhanced expression of membrane-bound CD163 on circulating monocytes of asthmatic patients treated with systemic corticosteroids [19]. The study indicates that systemic corticosteroid therapy of asthmatic patients leads to upregulation of monocyte CD163, which may be released as a soluble form at the site of monocyte recruitment. We have previously demonstrated that in asthmatic patients, the expression of CD163 on peripheral blood monocytes differs depending on the clinical status and monocyte subset [18, 19]. Asthma exacerbations, at least dependent on allergen exposure, seem to preferentially attract monocytes with a high CD163 expression [30]. This in turn may participate in downregulation of inflammatory response by releasing sCD163 in the airways. At least one enzyme, MMP-9, which participates in shedding of CD163 from monocyte/macrophage membranes is upregulated in the airways of asthmatic patients [31].
The elevated serum sCD163 concentration has also been reported in overweight subjects and the level correlates with BMI [20]. However, the mean BMI in our patients did not differ significantly in individual subgroups. Therefore, changes in the serum sCD163 concentration seem to reflect true relations that occur in asthmatic patients in vivo.
A tightly regulated expression of CD163 on peripheral blood monocytes and its release as a soluble form at the inflammatory site makes this molecule an attractive target which can act as a carrier for molecules which should be transferred to the particular site of the body [32]. Our research supports the concept, which assumes that CD163 might be able to carry through anti-inflammatory agents such as dexamethasone to the site of inflammation. Moreover, monocytes that express a high level of CD163 seem to be an interesting target of anti-inflammatory therapies in allergic and inflammatory diseases. Therefore, further studies to evaluate and fully understand its role in inflammatory diseases, such as asthma, are still a necessity.