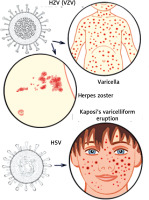
Introduction
Infections by pathogens, such as viruses and bacteria, are typically perceived as harmful, such as in cases of herpes (varicella) zoster [1] and herpes simplex virus infections [2]. The herpes (HZV) or varicella zoster virus (VZV) [3], which is a member of the α-herpesviridae subfamily, comprising a polyhedral capsid surrounded by a membranous envelope structure (Figure 1) [3], and is one of eight herpes viruses known to infect humans. It causes varicella (chickenpox) (Figure 1) [1], a disease most commonly affecting children, teenagers, and young adults, and herpes zoster (HZ) in adults, particularly the elderly. Herpes simplex viruses (HSV) [4] are categorized into two types: herpes type 1 (HSV-1, or oral herpes), is also a member of the α-herpesviridae subfamily, and its structure is composed of linear dsDNA, an icosahedral capsid with a spikey envelope (Figure 1) [4], and herpes type 2 (HSV-2, or genital herpes). Kaposi’s varicelliform eruption (VE), eczema herpeticum (Figure 1) [5], is a rare but severe disseminated infection, predominantly found accompanying HSV infections. It generally occurs at sites of skin damage [6], such as eczema, particularly, in severe atopic dermatitis (AD), occurring in about 3% of AD patients [5], long-term external use of steroids, or occasionally burns.
AD [7] is a complex pathology mainly characterized by immune response dysfunction [8], T helper type (Th)2 dominant conditions [9], overexpression of Th2 cytokines such as interleukin (IL)-4 and IL-13, which play a key role in type 2 inflammation [10], and lower interferon-γ (IFN-γ) protein production is also significant [11], as downregulation of Th1 [12] but lesional skin also shows a mixed type-1 and type-2 immune responsiveness [13]. AD patients also exhibit unbalanced dysbiosis of skin microbiota characterized by Staphylococcus aureus colonization [14, 15], an increased risk of developing bacterial and viral infections with a suspected low production of the antimicrobial peptide cathelicidin [16], and impairment of the skin barrier such as a deficit in filaggrin [5, 16]. These are deemed to be defects in functions of immune cells [17] such as dendritic cells (DC), natural killer (NK) cells, and regulatory T cells (Tregs) [18, 19]; in particular, the function of Tregs, which are responsible for initial response to infection [20], is important in these virus infections which play a pivotal role in immune regulation and are integral to the control of allergic responses [21]. Potential treatments can be designed to amplify these cells to suppress the allergic inflammatory cascade in AD [21]. Atopic conditions are aggravated as a result of insufficient IL-12 production by DCs [22]. Acquired functional impairment of Tregs in AD patients and the correlation between the increased frequency of Tregs and disease severity support their important role in AD pathogenesis [23].
Alternatively, clinical observation of an improvement in severe atopic skin lesions after the onset of varicella has been noted [24], the improved state continued for at least 2 to 3 months and up to 5 to 6 months. In my daily dermatological practice, the author has occasionally observed improvements associated with HZV or HSV infections, especially in those with Kaposi’s VE (eczema herpeticum) in AD patients. I have earlier reported remarkable improvement in the eczematous lesions of both axillar regions that caused Kaposi’s VE in an elderly patient [25] with senile atopy and a secondary erythroderma due to a predisposition to atopy. It took considerably longer for skin lesions at other sites to heal. So far, the author has noted that protective responses to these infectious agents are expected to improve atopic eczema. Based on these clinical observations, this study focuses on Th1 immunoregulatory events caused by infectious pathogens, particularly herpes viruses.
Immunoregulatory events in HZV infection
Varicella (Figure 1) is a primary infection of VZV, which becomes latent in the peripheral ganglia [1]. However, declining T cell immunity [26] in ageing individuals or those undergoing immune restrictive treatments can lead to VZV reactivation and the development of herpes zoster (Figure 1) [1]. Varicella immunization can provide the opportunity to analyse the kinetics of IL-10, IL-12, and IFN-γ production [27] elicited during primary in vivo sensitization with VZV proteins [28]. VZV antigens on infected cells may be processed by monocytes for presentation to T cells [29]. In our previous reports, we found that monocytosis [30, 31], suggested as part of the defence against infection, was observed in the leukocyte fraction of the peripheral blood, which is typically composed of 2–10% of all leukocytes in the human body. Monocytes play a role with their diverse functional properties to protect infection in mobilizing dendritic cells (DCs) [32]. Monocytes derived from DCs, which are recruited during infection defence, produce large amounts of IL-12 [33]. Monocytes serve multiple roles in immune function and are the largest type of leukocyte and can differentiate into macrophages and myeloid lineage DCs, characterized by a high level expression of the CD14 cell surface receptor (CD14+ CD16++ monocyte) [34]. CD14 [35] is a human monocyte differentiation antigen as the Toll-like co-receptor for the detection of pathogen-associated molecular patterns. After stimulation with microbial products, the CD14+CD16++ monocytes [34] produce high amounts of pro-inflammatory cytokines like tumour necrosis factor (TNF) and IL-12. IFN-γ is also released upon activation of Th1 NK cells [36], which take the role of the innate immune defence against infection [37], during protection against viral infections. It is assumed that Th1 induction is involved in the suppression of Th2 atopy conditions through this process. Although studies of immunological processes have advanced, monocyte immune function and the positive aspects of the human immune response system caused by viruses have not been widely examined. Moreover, IL-12 was discovered as a key immunoregulatory cytokine in various infections and is effective in fighting a wide range of viral infections [38, 39] and may promote a Th1 response and regulate Th1 stability [40]. As shown in our atopic mouse experiments with bacterial components of Streptococcus pyogenes [41], IL-12 was amplified. It has been confirmed that depending on these bacterial infections, an IL-12 inducer of the defence process exerts effects on Th1 conditions. In human varicella infection, Fujimura et al. [24] showed that a switch from Th2 to Th1 regulation occurred, supporting observations of atopic dermatitis improvement after varicella infection. Thus far, HZV may have contributed to the switch to Th1 dominance [24]. In such lesions, expression of Th1 type cytokines predominated [24], suggesting downregulation of Th2 dominant AD conditions. They also pointed out that IL-12 may regulate the switch of the recall response of allergen-specific T cells of atopic donors from a Th2- to a Th1-like phenotype in vitro [24]. Although the detailed mechanisms remain unclear, immunity is known to decline with age [42] and in those with cancer or various infectious diseases, and the side effects of drugs [43] are often observed in the elderly. These accumulated findings suggest that the deficiency in the immune response due to ageing and diseases such as atopy can be repaired and normalized despite a temporary immune response to protect against infection caused by HZV.
Immunoregulatory events in HSV infection
In HSV infections, the type I interferon (INF-α and β) signalling pathway [44] plays an important role in the innate immunity [45, 46] along with activated neutrophils, monocytes, macrophages, and dendritic cells (DCs). DCs [47] are antigen presenting cells that are important for pathogen recognition at sites of infection and for priming of protective HSV-specific T cells [48]. NK cells [49] play an important role in the host response against viral infections being able to kill virus-infected cells. The control of Tregs may also maintain the delicate balance between inflammation and healing in controlling HSV infections [18]. It is likely that children with AD may be susceptible to HSV due to reduced numbers of NK cells and a decrease of IL-2 receptors, a marker for lymphocyte activation, during early eczema herpeticum [50]. HSV infection in vitro also has been found to up-regulate the expression of IL-12 (p40) mRNA as a triggering event that biases HSV-specific immunity to a type 1 T cell response [51]. Patients with Kaposi’s VE have impaired the type II interferon (IFN-γ) production in the protective response to HSV [52]. The impaired INF-γ production may account for the abnormal immunopathogenesis of severe, intractable AD [11]. Patients with Kaposi’s VE (Figure 1) exhibit reduced IFN-γ production, which may contribute to an impaired immune response to HSV [11, 52]. Of note, some reports suggest that HSV increases the levels of Th2 cytokines, such as IL-4 [53] and IL-25 [54], which, in turn, promote HSV replication; in fact, IL-25 [54] was shown to promote HSV replication by inhibiting the expression of filaggrin, suggesting this protein as an aggravating factor in Kaposi’s VE. It has also been pointed out that CD14(dim)CD16(+) monocytes [20] have a compromised ability to produce pro-inflammatory cytokines. Therefore, Kaposi’s VE is attributed to a decrease in the immune function of the skin of AD patients [8]. Nevertheless, improvement in AD skin lesions of Kaposi’s VE cases suggests that immune response to HSV infection involves a specific immunomodulatory activating factor. On the whole, this field has not always been actively investigated. The authors have experimented with animal models to scientifically support an observed clinical event. We have previously reported an improvement in AD-like lesions by UV-inactivated HSV 1 [55] in a murine atopy model (Nc/Nga mice), supporting a remarkable improvement in eczema lesions at the site of Kaposi’s VE. On the other hand, Kawakami et al. [56] described a relationship between defective NK cell activity and development of HSV 1-induced severe skin lesions (eczema herpeticum) in eczematous Nc/Nga mice. Herpes virus disease symptoms in patients with deficiencies in NK cell activity may result in life-threatening conditions [49]. Human plasmacytoid dendritic cells (pDCs) in the activation of NK cells control such herpes virus infections [47]. So far, NK cell activation [17, 47] could be induced by HSV in some individuals with improved atopic skin lesions. The difference between our previously reported results [55] and those of Kawakami et al. [56] is likely due to the use of different methodologies. In our study [55], 2 × 105 pfu UV-inactivated HSV 1 per mouse was injected intra- and/or subcutaneously at six sites in the eczema skin lesions. Contrarily, in Kawakami’s experiments [56], 4.5 × 103 pfu (in a volume of 3 μl per site) of live HSV 1 per site was intradermally injected at four sites in the skin lesions. Although the dose of virus administered in the studies differed by a factor of 10, the live and inactivated viruses induced completely different immune responses. Furthermore, there may be some difficulty in deciding on the appropriate dosage of live virus for small laboratory animals. Dose-dependent differences in immune response must also be taken into account when comparing different studies [57]. We hope to further analyse the mechanism of eczema lesion improvement in Kaposi’s VE using the response against HSV infection.
Prospects of Th1 regulation by infectious pathogens
In our previous studies [41, 55], we have shown through animal experiments that allergic dermatitis can be alleviated not only by these viruses [55] but also by S. pyogenes components, such as OK-432 [41]. Findings [58] in support of this result have already been shown. These pathogens may have components that induce immunological events contributing to Th2 to Th1 shifts [41]. While the severity of the immune response in infectious diseases is important, humanity has succumbed to numerous infectious agents despite the development of vaccines [28]. Although the results of animal experiments cannot be immediately reproduced in humans, they have been evaluated continuously to increase their applicability in humans later on. Therefore, we will continue to study the benefits of the immune response in some infectious pathogens. There is also a worldwide need for immunological studies to ascertain the components of these pathogens that can suppress or improve the symptoms of allergies. If these beneficial components of pathogens are found, it may be possible to replenish and improve atopic and age-related immune dysfunction and maintain patients in a stable condition. Further advances in this field will require identification of the molecular components of pathogens [59] that determine the direction of immune response and act as suppressors of allergic dermatitis. Lundberg et al. [59] reported that HSV DNA or HSV-derived oligodeoxyribonucleotides (ODNs) can induce the production of inflammatory cytokines, such as IFN-γ, which regulate Th1 response. Moreover, the augmentation of Th1 responses by bacterial cellular components such as lipopolysaccharides (LPS) in Gram-negative bacteria and peptidoglycans and lipopeptides in gram-positive bacteria, has also been highlighted [59]. As our study using components of S. pyogenes showed, lipoteichoic acid-related molecules [60] induced improvement of AD-like skin lesions in Nc/Nga mice. AD cases effectively treated with S. pyogenes bacterial extracts, such as OK-432 [61], have been previously reported. T lymphocytes recognize VZV glycoproteins (gpI-V), the immediate early/tegument protein, and the product of gene 62 (IE62) [62]. Thus far, identification of herpes virus components regulating such Th1 shifts are yet to be elucidated. Alternatively, the attenuated VZV vaccine (Oka/Merck) and inactivated zoster vaccine, prepared by heat or irradiation, recruit amplification of VZV-specific T-cell mediated responses with the enhancement of the vaccine-induced IFN-γ [63]. It may be worthwhile not only to use vaccines as protection against infection, but also for immunostimulation with Th1 induction. This may be effective in elderly people [42, 63], whose immune response has declined due to age. In atopy patients [64], as reported by Foerster and Molêda [65], vaccine targeting IL-13, a Th2 cytokine, may prove beneficial.
Conclusions
From the accumulated findings discussed here, both HZV and HSV may induce Th1 regulatory events along with the protective immune response to infection, although HZV appears to induce more systemic immunostimulation. HSV appears to be a local response in affected skin lesions. To date, great progress in immunopathological analysis in allergic diseases and AD has been made, but much work remains to elucidate the mechanism underlying this phenomenon and establish a reliable and safe treatment strategy. Induction of Th1 response by these viruses should be evaluated to improve the complex immune responses induced by the mobilization of Th1-regulating cytokines such as INF-γ and IL-12, to improve the symptoms of Th2-dominated AD. Although it is also true that these viral infections are nearly always harmful, there are rare cases in which atopic eczematous lesions improve upon exposure to these viral infections. However, it is possible that immune responses to the infectious agent may vary among individuals. Analysis of the immune events induced by these viral infections would provide novel insights for atopic therapy. It seems reasonable to suppose that infectious pathogens are not necessarily all bad, and further analysis in this field is required to uncover the potential therapeutic benefits of some pathogens.