Introduction
Psoriasis is an immune-mediated skin disorder with a prevalence of 2% in Europe and North America [1]. People suffering from psoriasis are at increased risk of developing additional serious conditions such as cardiometabolic diseases, malignancies or depression. Moreover, approximately 20% of patients with psoriasis will develop psoriatic arthritis at some point in their lifetime [2]. For mild cases of psoriasis, the main treatment approach involves topical therapy, whereas patients with moderate to severe psoriasis necessitate more advanced treatment options like phototherapy, retinoids, or traditional immunosuppressants [3]. Nevertheless, a significant number of individuals with psoriasis experience inadequate treatment outcomes with initial systemic therapies or have to discontinue therapy due to adverse events associated with treatment or comorbidities.
One of the major advancements in the treatment of psoriasis was the introduction of biologic agents. This breakthrough stemmed from understanding the disease’s pathogenesis, which involves immune system dysregulation leading to uncontrolled proliferation and abnormal differentiation of keratinocytes. Primarily, the discovery that the development of psoriatic plaques might be triggered by cytotoxic T lymphocytes producing effector cytokines (interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α)) prompted the utilization of TNF-α inhibitors (like infliximab, adalimumab, etanercept, certolizumab pegol) in treating psoriasis [4, 5]. Following this, the identification of T helper 17 (Th17) cells and their secretion of interleukin-17 (IL-17) and IL-22 emerged as additional crucial factors in the development of psoriasis by triggering the epidermal changes [6, 7]. Upstream of this process, dendritic cells secrete IL-23 to stimulate the differentiation and proliferation of Th17 cells and IL-12, which induces the production of IFN-γ, required for the development of Th1 immune response [8, 9]. These discoveries have led to the development of new, highly effective generations of biologic drugs – inhibitors of IL-23 and related cytokines (ustekinumab, guselkumab, tildrakizumab, risankizumab) and the inhibitors of the IL-17 pathway (secukinumab, ixekizumab, brodalumab, bimekizumab). With the use of biologics, it is now feasible to achieve total or nearly total clearance of skin lesions and alleviate inflammatory joints symptoms. Nevertheless, their immunomodulatory mechanism of action raises concerns about potentially increased risks of infections and malignancies.
The risk of cancer in psoriatic patients has been a topic of ongoing discussion. It may be increased by the common lifestyle habits, such as smoking or alcohol consumption. Moreover, there has been speculation regarding the association between systemic treatments like phototherapy and immunosuppressive agents with cancer [10, 11]. The pivotal issue is the risk of skin cancer in patients with psoriasis attributed to their exposure to ultraviolet radiation and the usage of immunosuppressive drugs. According to studies, there is an increased risk of melanoma and non-melanoma skin cancer (NMSC) in patients treated with TNF-α inhibitors [12, 13]. Less is known about the risk of skin malignancies in patients treated with newer biologics (IL-23 inhibitors, IL-17 inhibitors); however, recently published meta-analysis suggested that the risk of melanoma and NMSC may be lower in these populations than in patients treated with TNF-α inhibitors [14].
The aim of this study is to explore the role of IL-12, IL-23 and IL-17 in the development of skin neoplasms and to review the data on safety of novel biologics (IL-12 inhibitors, IL-23 inhibitors, IL-17 inhibitors) concerning the risk of melanoma and NMSC.
The role of IL-12 and IL-23 in the tumorigenesis
Both IL-12 and IL-23 belong to the IL-12 cytokine family, comprising IL-12, IL-23, IL-27, and IL-35 [15]. They are produced by dendritic cells and macrophages in response to various exogenous and endogenous stimuli. IL-12 and IL-23 exert primarily proinflammatory effects and play crucial roles in promoting the development of Th1 and Th17 cells, respectively. IL-12 comprises two subunits: IL-12p40 and IL-12p35. When combined as a heterodimer, it interacts with the IL-12 receptor, consisting of IL-12Rβ1 and IL-12Rβ2 subunits. Downstream signalling occurs via the Janus kinase–signal transducers and activators of transcription 4 (JAK-STAT4) pathway, through which IL-12 facilitates the differentiation of naïve CD4 T cells into Th1 cells that produce interferon-γ (IFN-γ) [16, 17]. IL-23 consists of the IL-23p19 subunit, along with the IL-12p40 subunit (shared with IL-12), and it signals through both the IL-23 receptor and the IL-12Rβ1 subunit. IL-23 utilizes JAK-STAT3 and JAK-STAT4 pathways to enable the transformation of naïve T cells into Th17 cells producing interleukin-17 (IL-17) [16, 17].
In the majority of preclinical models, IL-12 has demonstrated strong efficacy in stimulating anti-tumour responses [18]. The anti-tumour properties of the IL-12-IFN-γ axis were initially demonstrated in models where exogenous IL-12 were administered to tumour-bearing mice. IL-12 treatment substantially increased survival rates and resulted in tumour remission [19]. Another model showed that IL-12 can inhibit tumour induction by 3-methylcholanthrene, a carcinogenic hydrocarbon, in mice [20]. As demonstrated in murine models, IL-12 exhibits strong anti-tumour activity by inducing the production of IFN-γ and activating effector cells like CD8+ T cells and NK cells [21]. Additionally, according to Eisenring et al., IL-12 triggers local anti-tumour immunity by activating a subset of NKp46+ lymphoid tissue inducer cells, which relies on the transcription factor RORγt. This activation leads to an enhanced expression of adhesion molecules within the tumour vasculature, thereby promoting increased infiltration of leukocytes into the tumour site [22].
Despite extensive research efforts in recent years, the precise role of IL-23 in cancer remains uncertain. Nonetheless, the majority of studies suggest that IL-23 primarily functions as a promoter of carcinogenesis. The significance of IL-23 in tumorigenesis was illustrated in experiments involving mice deficient in IL-23p19. These mice displayed near-complete resistance to skin papilloma formation induced by carcinogen 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA) [23]. In alternate carcinogenesis and metastasis model mice lacking IL-23 showed resistance to experimental tumour metastases and the absence of IL-23 enhanced the antitumor effector function of perforin and IFN-γ. Notably, in carcinogenesis model IL-23 deficiency provided remarkable protection against tumour formation [24].
The role of IL-12 and IL-23 in the development of skin neoplasms
The anti-tumour action of IL-12 in inhibiting the development of NMSC has been explored in animal models and shows conflicting results. Schwarz et al. discovered that IL-12 led to a significant decrease in UV-induced DNA damage by stimulating DNA repair mechanisms, consequently hindering the progression of skin cancer [25]. Additionally, IL-12 induced the expression of specific components within the nucleotide-excision repair system and prevented UV-induced systemic immunosuppression [25–27]. Similarly, the role of IL-23 in the UV-induced immunosuppression has been investigated and, according to the studies, administering IL-23 to mice exposed to UVR reduced both the number of apoptotic keratinocytes and the levels of DNA damage [28].
A skin carcinogenesis model was employed by Langowski et al. [23], who utilized a genetic deletion approach to investigate the susceptibility of mice lacking either IL-12 or IL-23 (p35 or p19 lacking mice) or mice deficient in both cytokines (p40 lacking mice) to tumour formation during carcinogenesis. Mice lacking IL-23 were resistant to tumour induction, indicating the potential role of IL-23 in promoting the growth of skin cancer. Moreover, IL-23 lacking mice were characterized by increase in infiltrating CD8+ T cells and reduced levels of IL-17, matrix metallopeptidase 9 (MMP9) and CD31 expression, what emphasizes the involvement of MMP9 and other genes related to angiogenesis in IL-23 promoted carcinogenesis [23]. Additional experiments supported this view. For example, according to Kortylewski et al., signalling through Stat3 within the tumour microenvironment triggers the production of IL-23 while simultaneously suppressing IL-12, what promotes carcinogenesis [29].
Since melanoma is highly immunogenic tumour, many researchers are focusing on the role of immune and inflammatory mechanisms in melanoma pathogenesis and prognosis. The role of IL-12 and IL-23 in the development of dysplastic nevi, melanoma and metastases has been examined by Nasti et al. [30]. In an animal model where mice develop numerous pigmented nevi progressing into invasive melanomas, IL-23p19-deficient mice exhibited 70% more nevi that grew faster, leading to larger melanomas with a higher likelihood of metastasis. Conversely, IL-12p35-deficient mice developed fewer melanocytic tumours compared to wild-type mice. Another observation was that melanocytic cell lines derived from IL-12p35-deficient mice had fewer H-ras mutations compared to those from IL-23p19-deficient mice and wild-type mice. Additionally, they observed enhanced DNA repair in IL-12p35-deficient mice. According to the study, IL-23 was discovered to be crucial for maintaining melanocyte homeostasis and inhibiting melanoma progression by direct activation of DNA repair in melanocytes, whereas IL-12 promoted the development of nevi [30]. The discovery above seems to conflict with previous reports on the roles of IL-12 and IL-23 in carcinogenesis. In accordance with the above, Overwijk et al. discovered that IL-23 functions as a strong adjuvant in vaccines, promoting the generation of tumour-specific CD8+ T cells, causing tumour destruction and growth suppression [31].
Fang et al. discovered a correlation between elevated IL-12p40 blood levels and a poorer prognosis in early-stage melanoma patients. The study suggests that therapies targeting IL-12 and related cytokines could potentially be beneficial for individuals with melanoma [32].
Safety of IL-12 and IL-23 inhibitors in clinical studies
Ustekinumab, an anti-IL-12/23p40 antibody, has been approved for treating moderate to severe psoriasis since 2009. Consequently, its safety has been fairly extensively studied compared to other newer biologics. According to two large clinical studies evaluating the efficacy and safety of ustekinumab – PHOENIX 1 and PHOENIX 2 studies – the risk of overall malignancies during 5 years of follow-up was 0.93 and 1.08 per 100 patient-years (PY), respectively, and the risk of NMSC was 0.45 and 0.42 per 100 PY, respectively [33, 34]. In a recently published systematic review and meta-analysis analysing the risk of melanoma and NMSC in patients with psoriasis and psoriatic arthritis, the risk of NMSC in subjects treated with ustekinumab was 0.1 per 100 PY, while the risk of NMSC in overall patients treated with targeted therapies was 0.45 per 100 PY [14]. Considering that the age-standardized incidence rate of NMSC in the general population was recorded as 0.08 per 100 in 2019, the risk associated with the use of ustekinumab does not appear to be significantly elevated [35].
Among the anti-IL-23p19 antibodies, three are being widely used for the treatment of rheumatoid diseases: guselkumab, tildrakizumab and risankizumab (years of approval: 2017, 2018, 2019, respectively) [36]. IL-23 inhibitors represent a newer class of drugs and require further research to establish their long-time safety; nevertheless, certain studies suggest their relative safety in terms of skin neoplasms. The pooled results from the VOYAGE 1 and VOYAGE 2 trials showed that the overall malignancy rates in guselkumab-treated population were low and consistent with rates in both the general population and among those with psoriasis. Among the 1721 patients receiving guselkumab, 4 cases of melanoma and 24 cases of NMSC were noted [37]. Similar findings have been observed in the pooled analysis from reSURFACE 1 and reSURFACE 2 clinical trials: in 1800 patients, 5 cases of melanoma and 23 cases of NMSC were observed [38], while in the LIMMitless study evaluating long-term safety of risankizumab among the 897 patients, 3 cases of melanoma and 18 cases of NMSC during 5 years of follow-up were noted [39]. Based on the meta-analysis conducted by Krzysztofik et al. [14], the overall risk of melanoma and NMSC in patients treated with IL-23 inhibitors was 0.1 per 100 PY and 0.49 per 100 PY, respectively. This indicates a slight elevation in the risk of both melanoma and NMSC in comparison to the general population rates, which stand at 0.02 per 100 PY and 0.08 per 100 PY for melanoma and NMSC, respectively [35, 40]. Additionally, in a recently published study, treatment with IL-23 inhibitors was associated with a reduced risk of BCC when compared to TNF-α inhibitors, and reduced risk of BCC and SCC when compared to the biologic-naïve patients [41].
The role of IL-17 in the tumorigenesis
The IL-17 family of cytokines comprises IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F, which share structural similarities among themselves. Correspondingly, the IL-17 receptor (IL-17R) family consists of IL-17A, IL-17B, IL-17C, IL-17D and IL-17E subunits. IL-17F, bearing a 55% overlap with IL-17A, is notably the most homologous cytokine to IL-17A. IL-17A and IL-17F have the capability to create homodimers or heterodimers with each other in both humans and mice and bind to a common receptor complex composed of IL-17RA and IL-17RC subunits [42, 43]. IL-17A primarily originates from a subset of T helper cells called Th17 cells, along with γδT cells, both of which also produce IL-17F [44]. The initial evidence of IL-17’s pro-inflammatory function was established in fibroblasts, demonstrating its ability to activate nuclear factor k-light-chain-enhancer of activated B cells (NF-κB) and trigger NF-κB-dependent cytokines [45, 46]. Further investigations have outlined a distinct IL-17 core gene profile, comprising pro-inflammatory cytokines (IL-6, granulocyte-colony-stimulating factor, TNF-α), chemokines, antimicrobial proteins (defensins, mucins), matrix metalloproteinases and inflammatory effectors [47].
IL-17 has been detected in diverse tumours and demonstrates dual roles, exhibiting both pro-tumour and anti-tumour effects, influenced largely by the immunogenicity and cell type of the tumours [48]. The procarcinogenic effect of IL-17 is primarily associated with its promotion of angiogenesis within the tumour. In immunocompromised mice, IL-17 has been shown to enhance non-small cell lung cancer’s vascularity by stimulating CXCR-2-dependent angiogenesis [49]. Additionally, research has revealed that IL-17 stimulates production of proangiogenic factors (vascular endothelial growth factor (VEGF), keratinocyte-derived chemokine, macrophage inflammatory protein-2 and nitric oxide) in fibroblasts and promotes formation of new vessels induced by fibroblasts in both inflammatory conditions and tumours [50, 51]. The secretion of VEGF is stimulated by the direct action of IL-17 and its cooperation with TNF-α [52]. In a study conducted on human colorectal cancer cells, IL-17A, IL-22, TNF-α and IL-6, abundantly secreted by tumour-infiltrating leukocytes, enhanced STAT3/NF-κB activation and led to tumour progression [53].
On the other hand, the presence of IL-17 is associated with a better prognosis among patients with various cancers. For example, individuals diagnosed with chronic lymphocytic leukemia demonstrated a positive correlation between elevated circulating Th17 levels and more favourable prognostic markers and longer overall survival [54]. According to the study, Th17 cells could potentially influence CLL cells either directly or indirectly through interactions with T cells and monocytes, by inhibiting the proliferation of leukemic cells, suppressing angiogenesis, or facilitating the apoptosis of CLL cells. Furthermore, according to Lu et al., IL-17A has the potential to stimulate esophageal squamous cell carcinoma cells to secrete chemokines such as CXCL9, CXCL10, CCL2, and CCL20, which are responsible for the recruitment of T cells, NK cells, and dendritic cells. Moreover, IL-17A enhances the ability of NK cells to destroy tumour cells by increasing the production of cytotoxic molecules like TNF-α, IFN-γ, perforin, and granzyme B. It also promotes the activation of receptors such as NKp46, NKp44, NTB-A, and NKG2D on NK cells, further enhancing their cytotoxic activity against tumour cells [55]. Furthermore, the beneficial impact of IL-17F in colon cancer development has been demonstrated. In a study led by Tong et al., the overexpression of IL-17F hindered the in vivo proliferation of HCT116 cells, a human colon carcinoma-derived epithelial cell line, potentially through the suppression of tumour angiogenesis [56].
The role of IL-17 in the development of skin neoplasms
Despite several research studies, the precise role of IL-17 in melanoma remains uncertain, as conflicting reports exist regarding its capacity to either promote or inhibit tumour development. Martin-Orozco et al. [57] discovered that IL-17A and Th17 cells exhibit a protective function against lung melanoma cells by initiating a defensive inflammatory reaction to cancer. According to the study, Th17 cells promote the migration of dendritic cells to tumour sites, leading to an accumulation of CD8+ T cells carrying tumour antigens in draining lymph nodes. Additionally, Th17 cells notably stimulated the expression of CCL20 within tumour tissues. It has been also demonstrated that Th17 cells may provide protection against skin melanoma. However, the suppression of melanoma growth appeared to depend on IFN-γ, whereas depletion of IL-17A and IL-23 had a minimal effect [58]. The anti-tumour role of IL-17 signalling has been also shown by Rodriguez et al. [59]. According to this study, IL-17 signalling played a crucial role in facilitating the infiltration of tumour-specific CD8+ T cells in melanoma B16.SIY cell line. Correspondingly, Nuñez et al. found that Th17 cells facilitate the recruitment of effector Th1 cells to the tumour and, therefore, promote anti-tumour responses [60].
On the other hand, there is some evidence that IL-17 may influence melanoma progression. In a study, the suppression of IL-17A receptor in murine melanoma cells resulted in decreased cell proliferation and migration, along with a reduction in the production of VEGF and MMPs [61]. Similarly, the studies on the B16 melanoma cell line demonstrates that Th17 responses can potentially facilitate tumour growth through the induction of IL-6 production, which, in turn, activates the oncogenic STAT3, leading to the upregulation of genes associated with cell survival and angiogenesis [62, 63]. According to Rodriguez et al., whether IL-17 acts as a pro-tumour or anti-tumour factor depends on the tumour’s immunogenicity. The studies indicating a pro-tumour effect of IL-17 involved tumours with lower immunogenicity compared to those demonstrating an anti-tumour effect, highlighting the tumour microenvironment as a crucial factor for IL-17’s anti-tumour functions [59].
The findings from studies conducted on human samples are also inconsistent. One study on human-derived skin malignancies indicated a lower occurrence of IL-17A-expressing tumour-associated lymphocytes in cutaneous melanoma compared to basal cell carcinoma (BCC) [64]. Conversely, another study reported increased immune activation along the Th1/Th2/Th17 pathways during the transition from common melanocytic nevi to dysplastic nevi to malignant melanoma [65].
To date, little is known about the function of IL-17 in the development and progression of NMSC. The majority of existing research suggests that IL-17 facilitates the development of skin cancer rather than hindering it. Nardinocchi et al. [66] discovered that both BCC and squamous cell carcinoma (SCC) are infiltrated with a high number of IL-17+ and IL-22+ T lymphocytes, which was linked to enhanced tumour progression. IL-17 was capable of stimulating the production of two cytokines crucial for tumour advancement, namely IL-6 and IL-8, and upregulated NF-κB signalling [66]. Other studies have provided additional evidence supporting the potential pro-tumour role of IL-17. For instance, Wu et al. [67] identified a novel IL-17-mediated cascade through the IL-17R–Act1–TNF receptor-associated factor 4 – mitogen-activated protein kinase kinase kinase 3 – extracellular signal-regulated kinase 5 positive circuit, which promotes keratinocyte proliferation and tumour formation, while Tuong et al. [68] discovered that the levels of different pro-inflammatory cytokines, including TNF-α, IL-12p70, and IL-17A were significantly elevated in actinic keratosis and SCC lesions compared to normal skin. Similarly, levels of nitric oxide, IL-10, IL-17, TNF-α and TGF-β were elevated in the chemically induced SCC development model [69]. In a study conducted on human BCC samples, elevated levels of IFN-γ, IL-17, IL-23, and IL-22 were detected in BCCs, in comparison to healthy skin. IFN-γ was more abundant in superficial BCCs in contrast to nodular BCCs, where IL-17 levels were elevated [70].
Safety of IL-17 inhibitors in clinical studies
Four human monoclonal antibodies are approved for treating psoriasis: secukinumab and ixekizumab (IL-17A inhibitors), brodalumab (an inhibitor of the IL-17A receptor subunit), and the most recent antibody, bimekizumab (an inhibitor of both IL-17A and IL-17F). While secukinumab and ixekizumab antagonize only the IL-17A cytokine, brodalumab and bimekizumab also disrupt IL-17F action [71]. The safety profile of IL-17 inhibitors concerning the risk of skin neoplasms has been detailed in clinical trials. In the FUTURE 2 study, which assessed the safety and efficacy of secukinumab among patients with psoriatic arthritis, 3 cases of BCC and 3 cases of SCC of the skin were reported among 387 patients [72]. Another analysis found that the incidence of skin cancer among psoriatic patients treated with ixekizumab remained low and did not increase with longer exposure. Among 6892 patients, 44 had BCC, 16 had SCC, and 2 had melanoma [73]. Brodalumab, in a pooled analysis of five clinical studies, also demonstrated a comparably low incidence of skin cancer, with 39 cases of BCC, 15 cases of SCC, and 1 case of basosquamous carcinoma among 4464 patients [74]. Bimekizumab, the newest IL-17 inhibitor that targets both IL-17A and IL-17F, showed a similar safety profile. In the BE OPTIMAL study, 3 cases of BCC and 1 case of SCC were identified among 702 patients with psoriatic arthritis [75].
According to the meta-analysis, the incidence rate of melanoma events per 100 patient-years (PYs) was 0.06 (95% CI: 0.02–0.18), and the risk of NMSC events per 100 PYs was 0.19 (95% CI: 0.05–0.68) among patients treated with IL-17 inhibitors. Importantly, these risks were lower compared to patients treated with IL-23 inhibitors [14]. Additionally, when compared to both biologic-naïve patients and those treated with TNF-α inhibitors, individuals treated with IL-17 inhibitors showed a reduced risk of melanoma and BCC, but not SCC [41].
Conclusions
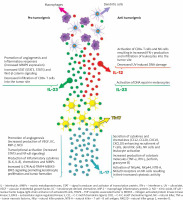
The well-established pro-inflammatory effects of IL-23/Th17-related cytokines are recognized for their significant involvement in immune-mediated inflammatory skin diseases. However, their role as either promoters or inhibitors of tumours in cancer remains uncertain based on preclinical research, which has shown contradictory findings regarding their impact on carcinogenesis (as summarized in Figure 1). Given the current clinical evidence, it can be hypothesized that targeting IL-17 and IL-12/23 with existing biologic treatments should not increase the risk of skin tumour development in individuals with moderate to severe psoriasis or psoriatic arthritis. Nevertheless, large-scale studies or pooled analyses with extended follow-up periods are needed to further validate this hypothesis.
Figure 1
Proposed model of the role of IL-23/Th17 pathway in carcinogenesis. Pro-tumorigenic effects are due to enhanced molecular signalling, increased angiogenesis, and elevated production of inflammatory cytokines, chemokines, and MMPs. On the other hand, anti-tumorigenic effects are associated with the activation of anticancer immune responses and cytotoxicity