Introduction
Obstructive airway diseases like asthma and chronic obstructive pulmonary disease (COPD) are progressive respiratory system diseases characterized by airflow limitation, airway inflammation, lung tissue remodelling, and airway wall damage [1, 2]. Obstructive airway diseases are showing a continuous increase globally and are considered a public health issue [3]. Due to their long-term progression and chronic course, obstructive airway diseases have a negative impact on patients’ quality of life [4, 5].
The treatment of obstructive airway diseases has consistently held the attention of respiratory physicians and researchers [6]. A broad selection of medications yields favourable results for patients, including montelukast sodium and budesonide/formoterol [7–9]. Montelukast sodium acts as a selective inhibitor of leukotriene receptors, lessening the action of leukotrienes to alleviate airway inflammation [10, 11]. In contrast, budesonide/formoterol comprises a fixed-dose combination of a protracted β2 adrenergic agonist and a prolonged anticholinergic medication, enhancing airflow by widening the airways to alleviate limitations [12]. A meta-analysis by Hon et al. found that montelukast is a useful treatment option for mild to moderate childhood asthma, particularly as monotherapy or add-on therapy to inhaled corticosteroids (ICS), with benefits in young children with viral-triggered wheezing diseases and exercise-induced asthma [13]. A meta-analysis of 9 studies found that the combination of montelukast and fluticasone propionate (Mon + Flu) was more effective than Flu alone in treating cough variant asthma in children, with significant improvements in the total effective rate, cough recurrence, and forced expiratory volume in 1 s (FEV1%) recovery. The study also found that Mon + Flu was safe and well-tolerated, with reduced cough remission and disappearance times compared to Flu alone [14]. A meta-analysis by Xu et al. found that montelukast as adjuvant therapy significantly improved the total effective rate, lung function, and reduced the recurrence rate in adults with cough variant asthma, compared to treatment with inhaled corticosteroids (ICS) and long-acting β2 agonists (LABAs) alone [15].
Aim
While prior studies have demonstrated the efficacy of different combinations of medications in treating obstructive airway diseases, we aimed to evaluate the effect of combining montelukast sodium with budesonide/formoterol on serum biomarkers like interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), eosinophils (EOS) count, which has not been extensively studied, in order to provide a more comprehensive understanding of its clinical implications and safety in the treatment of obstructive airway diseases.
Material and methods
General information
Research subjects for this study encompassed 100 cases of obstructive airway disease patients who received treatment at our hospital from January 2023 to December 2023. A prospective study was conducted on 100 obstructive airway disease patients who had received treatment of budesonide/formoterol inhalation powder (control group, n = 50) or montelukast sodium tablets supplemented to budesonide/formoterol inhalation powder (observation group, n = 50). The protocol was approved for implementation by our hospital’s ethics committee.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients fulfilled the clinical diagnostic criteria for obstructive airway diseases [16]; (2) There was no recent use of intravenous corticosteroids, leukotriene receptor antagonists, or β2 receptor agonists within 14 days before inclusion; (3) They were conscious and had effective communication abilities; (4) No known drug allergies; (5) They were free from severe organ diseases related to the heart, liver, or kidneys; (6) All patients were briefed on the study’s procedures and provided their informed consent voluntarily.
Exclusion criteria: (1) Subjects afflicted by malignant tumours; (2) Subjects with hypersensitivity to the experimental drugs; (3) Subjects who developed a respiratory system infection within 7 days before inclusion; (4) Subjects with inadequate clinical data; (5) Subjects experiencing consciousness disorders or mental impairments.
Intervention methods
The control group was administered budesonide/formoterol inhalation powder (II) (AstraZeneca AB), with each inhalation containing 160 μg of budesonide and 4.5 μg of formoterol, taken twice daily. The observation group was administered montelukast sodium tablets (Hangzhou MSD Pharmaceutical Co., Ltd.) in combination with the control group’s treatment, with a 10 mg oral dose taken once daily before bedtime. Both groups sustained this treatment for 3 months.
Evaluation criteria and observational indicators
Clinical efficacy assessment
The criteria governing the evaluation of therapeutic efficacy were devised based on the literature [17], and the progress of symptoms during treatment was assessed. Remarkable improvement was characterized by the disappearance of symptoms such as cough and expectoration within 3 days, with clear lung sounds upon auscultation; effective treatment involved a notable improvement in symptoms like cough, expectoration, wheezing, and dyspnoea within 7 days. Ineffectiveness was marked by an absence of changes in clinical symptoms post-treatment, or a deterioration of symptoms. The overall effectiveness was assessed as a percentage by calculating the addition of markedly improved and effective cases divided by the total cases.
Symptom improvement time
Evaluating the timeframe required for the amelioration of symptoms like cough, expectoration, wheezing, and dyspnoea in each group.
Pulmonary function monitoring
Using the MasterScreen SeS pulmonary function device, produced by Weinmann Medical Technology, the measurement of FEV1 and FEV1/forced vital capacity (FVC) was conducted. Greater values signify enhanced pulmonary function.
Measurement of serum and airway inflammatory mediators
Prior to and following the treatment, a venous blood sample (5 ml) was drawn from the patients in a fasting state, then centrifuged and preserved at –80°C for examination. The levels of IL-6 and TNF-α in the patient’s serum were evaluated via chemiluminescence. Utilizing the SYSMEX XN-2800 automated blood cell analyser (Sysmex), the eosinophil count measurement was performed. Moreover, the fractional exhaled nitric oxide (FeNO) was assessed through a fractional exhaled nitric oxide detector (Hefei Huigu Medical Technology Co., Ltd). The patient’s FeNO level serves as an indicator of airway inflammation and airway hyperresponsiveness, and was measured at a respiratory flow rate of 50 ml/s [18].
Results
The demographic characteristics of asthma/COPD patients in the intervention and control groups were comparable, as shown in Table 1. The mean age of participants was 58.2 ±10.5 years in the intervention group and 59.5 ±10.5 years in the control group, with no significant difference between the two groups (p = 0.54). The gender distribution was also similar, with 52% of the intervention group and 56% of the control group being male (p = 0.83). Additionally, the mean number of years since diagnosis were comparable between the two groups for both asthma (10.3 ±7.2 vs. 11.1 ±8.1, p = 0.71) and COPD (12.5 ±9.5 vs. 13.2 ±10.3, p = 0.71), indicating that the groups were well-matched in terms of demographic characteristics.
Table 1
Demographic characteristics of asthma/COPD patients by group
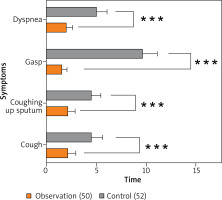
In the observation group, the time for improvement of symptoms including cough, sputum, wheezing, and dyspnoea was considerably shorter than that in the control group (p < 0.05) (Figure 1).
Subsequent to the treatment, there was an improvement in the FEV1, FVC, and FEV1/FVC for both groups of patients. The levels of these parameters were significantly higher in the observation group than in the control group (p < 0.05). Post-treatment, the serum levels of IL-6, TNF-α, FeNO, and EOS count decreased in both groups. The extent of improvement in these levels was significantly greater in the observation group than in the control group (p < 0.05). In the observation group, the total treatment effectiveness was 94.00%, showing a significant increase compared to the 80% observed in the control group (p < 0.05) (Table 2). No substantial disparity was observed in the collective frequency of adverse reactions between the control group and the observation group (p > 0.05) (Table 2).
Table 2
Study primary outcomes compared between the groups
| Group | Observation group (n = 50) | Control group (n = 50) | P-value | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| FEV1/l, mean ± SD | 1.53 ±0.32 | 2.20 ±0.41* | 1.50 ±0.35 | 1.92 ±0.41* | 0.681 | |
| FEV1/FVC (%), mean ± SD | 37.12 ±8.44 | 52.70 ±10.76* | 37.37 ±8.44 | 40.19 ±16.06* | 0.888 | |
| TNF-α [μg/l] mean ± SD | 13.23 ±1.42 | 4.18 ±0.41* | 12.65 ±1.62 | 8.25 ±0.96* | 0.059 | |
| IL-6 [μg/l] mean ± SD | 172.29 ±41.09 | 130.72 ±13.74* | 172.61 ±38.90 | 141.43 ±18.49* | 0.968 | |
| FeNO [ppb] mean ± SD | 45.50 ±5.07 | 19.80 ±3.31* | 43.84 ±4.78 | 33.45 ±3.78* | 0.094 | |
| EOS count (%), mean ± SD | 7.95 ±1.39 | 1.65 ±0.58* | 8.18 ±1.61 | 4.10 ±1.05* | 0.451 | |
| Efficacy, n (%) | Markedly effective | – | 30 (60.00) | – | 21 (42.00) | 0.037 |
| Effective | – | 17 (34.00) | – | 19 (38.00) | ||
| Ineffective | – | 3 (6.00) | – | 10 (20.00) | ||
| Adverse events, n (%) | Nausea | – | 2 (4.00) | – | 2 (4.00) | 0.749 |
| Rash | – | 2 (4.00) | – | 1 (2.00) | ||
| Headache | – | 1 (2.00) | – | 3 (6.00) | ||
Discussion
In our study the group receiving budesonide/formoterol inhalation powder supplemented with montelukast showed significantly faster improvement in symptoms and better treatment outcomes, including increased lung function and reduced inflammation, compared to the control group receiving budesonide/formoterol inhalation powder alone (p < 0.05). The observation group also had a higher treatment effectiveness rate (94.00%) and similar adverse event rates compared to the control group (80% and p > 0.05, respectively).
Our study’s findings are consistent with those of Dai et al., which also investigated the efficacy of adding montelukast to budesonide/formoterol in patients with bronchial asthma [19]. Both studies demonstrated that the combination therapy resulted in significantly improved treatment outcomes, including faster symptom improvement, increased lung function, and reduced inflammation, compared to budesonide/formoterol alone. Specifically, Dai et al. reported a higher total efficacy rate, improved daytime and nighttime symptom scores, and enhanced pulmonary function indexes in the combination therapy group, while our study found significantly better treatment outcomes, including increased lung function and reduced inflammation. The consistency of these findings across the two studies suggests that the addition of montelukast to budesonide/formoterol is a valuable therapeutic strategy for patients with chronic persistent asthma, and supports the conclusion that this combination therapy is worth clinical application.
Chen et al.’s study examined the impact on serum high-sensitivity C-reactive protein (hs-CRP), cancer antigen 125 (CA-125), and interleukin (IL)-6 levels, as well as quality of life and exercise tolerance [20]. Notably, both our and their studies found that the addition of montelukast to budesonide/formoterol inhalation powder resulted in significant improvements in clinical outcomes, including reduced inflammation and improved lung function, compared to budesonide/formoterol inhalation powder alone. However, our study specifically found that the combination therapy led to faster improvement in symptoms and greater reductions in serum IL-6, TNF-α, FeNO, and EOS count, whereas Chen et al.’s study reported lower serum levels of hs-CRP, CA-125, and IL-6. The consistency in findings between the two studies suggests that montelukast/budesonide formoterol powder inhalation may be a promising therapeutic strategy for COPD management. Researchers found that an allergen challenge in the airways leads to an increase in IL-6 and its soluble receptor, sIL-6R, which can trigger IL-6 trans-signalling and promote inflammation and lung dysfunction. The study’s findings suggest that IL-6 trans-signalling plays a key role in the inflammatory response to allergens in the airways, and may be a potential target for therapeutic intervention in diseases such as asthma [21].
Our study and the study by Fang et al. both investigated the efficacy of montelukast in combination with budesonide/formoterol inhalation powder and found similar results [22].
Conclusions
This study’s strengths include its prospective design, which allows for a thorough evaluation of the effectiveness and safety of montelukast sodium in conjunction with budesonide/formoterol in treating obstructive airway diseases. The study’s large sample size and comparative analysis of outcomes between the control and observation groups provide robust evidence for the treatment’s efficacy. However, a limitation of the study is its relatively short treatment duration of 3 months, which may not be sufficient to capture long-term effects or potential adverse reactions. Additionally, the study’s reliance on symptom improvement as the primary outcome measure may not fully capture the treatment’s impact on disease progression or quality of life. In conclusion, the study suggests that montelukast sodium in conjunction with budesonide/formoterol is a safe and effective treatment for obstructive airway diseases, offering improved symptom alleviation, pulmonary function, and reduced inflammation, and provides valuable insights for clinical management.