Introduction
Primary cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of lymphoproliferative disorders derived from CD4+ lymphocytes. Mycosis fungoides (MF) is the most common type. Cutaneous manifestations are generally the first sign of the disease, including erythematous, scaly patches, plaques, and tumorous lesions, as well as erythroderma. The aetiology of CTCL is not fully explained, although there are many hypotheses that implicate, for instance, occupational or environmental exposure, other forms of chronic antigenic stimulation, and viral infection. The annual incidence of CTCL varies between 0.4 to 1.0 per 100,000 people in the population. The prognosis of the disease in the majority depends on the extent of skin, blood, lymph node, and visceral organ involvement, along with advanced age and systemic extension.
The standard treatment is based on skin directed therapy (highly potent glucocorticosteroids, topical retinoids, mechlorethamine, phototherapy, radiotherapy) as well as systemic therapies, including extracorporeal photopheresis, bexarotene, methotrexate, biological response modifiers such as interferon-α, and allogenic stem cell transplantation.
It has been suggested that MF patients are at higher risk of developing other malignancies, especially haematological ones such as B-cell non-Hodgkin’s lymphomas [1, 2], melanoma [1], lung [3], colon [3], and urinary tract cancers [1], compared to healthy individuals. However, the increased risk of these neoplasm can be related to exposure to chemical carcinogens (chemical-related cancers, e.g. urinary bladder cancer), the potential aetiological factors of MF are still being discussed. Patients with a second malignancy preceded by MF occurrence are often exposed to phototherapy, local irradiation, and electron beam therapy, as well as chemotherapy. These therapies reveal the potential to generate an intense immune response resulting in chronic systemic inflammation. Moreover, they can induce direct or indirect DNA damage, which is also associated with increased risk of malignant transformation. Presumably, the second malignancy in CTCL patients can also develop due to the decreased cell-mediated immune response of T lymphocytes.
Aim
The main purpose of the study was to analyse the incidence of other malignancies in CTCL patients.
Material and methods
The aim of the study was to analyse the incidence of other malignancies in patients with CTCL. Among all those included in the study, 177 individuals were diagnosed with MF or Sézary syndrome (SS). The patients were enrolled to the study during their visits in the Outpatient Clinic and hospitalization in the Department of Dermatology, Venereology, and Allergology in Gdansk (Medical University in Gdansk) from January 2010 to November 2018. The MF/SS diagnosis was confirmed by histopathological examination. The following information were recorded: date of birth, age at onset of MF/SS, date of diagnosis, stage at diagnosis, and methods of treatment. The clinical database of the patients was checked to evaluate the coexistence of CTCL and other primary malignant neoplasms. Clinical characteristics are shown in Table 1.
Table 1
Clinical characteristics of patients with mycosis fungoides (MF) and Sézary syndrome (SS), and patients with co-existing cancers
Results
During the whole study 177 patients were diagnosed with MF/SS. There were 97 male and 80 female patients with a male-to-female ratio of 1.21 : 1. One co-existing cancer was diagnosed in 16.94% of CTCL patients (30 patients), and 2 co-existing other cancers were found in 1.13% (2 patients). In this case the male-to-female ratio was 1.28 : 1, with 18 and 14 patients, respectively. Taking into account the whole group of patients with co-existing cancers, the most common were basal cell carcinoma (BCC) and lymphomatoid papulosis, which were found in 15.63% of individuals (Table 2). The second was lung cancer, which affected 12.50% of patients, and the third was B-cell lymphoma, which accounted 9.38% of all neoplasms found in MF/SS patients. Nevertheless, when taking into account the distribution by sex (Table 3), the situation was slightly different [4–7]. For female patients the most common were BCC and breast cancer (14.29% independently), while for men the most common was lymphomatoid papulosis (22.22%). It should be highlighted that 2 co-existing cancers were found in one female and 1 male patient: kidney cancer and lymphomatoid papulosis, and B-cell lymphoma and teratoma malignum, respectively.
Table 2
The incidence (%) and site of other primary cancers in patients with MF/SS (n = 32)
Table 3
Cancer distribution by sex
Discussion
The results of our study have supported previous findings concerning increased risk of second primary malignancy development, especially BCC, Hodgkin’s lymphoma, chronic leukaemia, and lung cancer, in patients with MF and SS. Previous epidemiological data revealed that the factors associated with the increased risk of secondary solid tumours are: stage IV of the disease, the presence of lymphomatoid papulosis, and duration of the disease (more than 10 years). Furthermore, for the vast majority of the patients, secondary malignancies occurred within the first year of the diagnosis of MF (60%) [8]. According to Cengiz et al., older age, higher stage of MF, and the presence of lymphomatoid papulosis are factors associated with increased risk of the coexistence of two other malignancies besides MF [8]. In our study, only 2 patients with MF were diagnosed with co-existing kidney cancer and lymphomatoid papulosis, and B-cell lymphoma and teratoma malignum, respectively.
According to Goyal et al., patients with MF are at increased risk of developing second malignancies, particularly patients with advanced stage disease or tumours [9]. The results of our study do not support previous findings. The vast majority of the patients were diagnosed with 1A and 1B stage of mycosis fungoides (respectively, 54.84% and 19.35% of patients). Tumour stage and Sezary syndrome was diagnosed in 4 (12.9%) Patients.
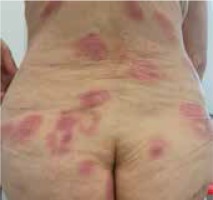
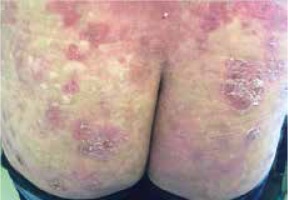
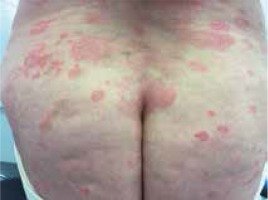
Moreover, we did not notice differences in the clinical picture in patients with concomitant malignancy (Figures 1–3).
Figure 2
A 67-year-old woman with folliculotropic mycosis fungoides and Hodgkin’s lymphoma (mixed cellularity subtype)
Furthermore, the incidence of malignancy was higher within the first year of diagnosis. In the study conducted by Amber et al. an increased risk of Hodgkin’s lymphoma development was demonstrated for men, and bronchopulmonary malignancies for women. Generally, their incidence was significantly higher in stage I and IV CTCL while the patients with CD30+ lymphocytes had a higher incidence of Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, and urinary cancers compared to the general population [10].
Interestingly, patients with MF were also characterized by higher risk of co-occurrence of cutaneous T-cell lymphoma with other hematoproliferative disorders. What is more, the risk of co-existing Hodgkin’s disease was highest for patients ≥ 60 years old. On the other hand, the risk of second non-Hodgkin’s lymphoma was highest in patients between 20 and 39 years of age [10].
In this study, a significant association with leukaemias and lymphomas was noticed in the MF group. A diagnosis of B cell lymphoma was found in 3 cases, while 1 patient was diagnosed with chronic lymphocytic leukaemia (CLL).
Additionally, we noticed that MF can be associated with acute leukaemia, specifically myeloid type. Our finding is supported by previous observations [11], but the reason for this association is not fully explained. Some authors suggest that this could be due to the exposure to the alkylating agents that may exert carcinogenic effects [12, 13]. In addition, several reports highlighted that the development of AML can be also associated with psoralen and ultraviolet A (PUVA) treatment [14, 15]. Nevertheless, we reported only 1 case of 41-year-old woman with AML and MF; however, the leukaemia was diagnosed a year before CTCL, and the patient had never received phototherapy.
This association of co-existence of Hodgkin’s lymphomas (HL) and CTCLs was described in 1963 for the first time. Approximately 40 cases have been reported in literature since that time [16]. In this study, the Hodgkin’s lymphoma was revealed in a 67-year-old woman who was diagnosed with MF 4 years earlier. Various treatments modalities were defined in her case, including topical steroids, UVB therapy, interferon α, and bexarotene, providing only partial and temporary improvements. Subsequently, the HL chemotherapy regimen ABVD (Adriamycin, Bleomycin, Vinblastine, Dacarbazine) was introduced (6 cycles).
It should be noted that the association of MF and HL has been described several times in the literature. Not only the genetic factors, but also the use of immunosuppressants in the treatment of this cutaneous lymphoma or Epstein-Barr virus infection have been implicated for the development of B-cell lymphoproliferative disorders [13, 17].
The results of this study also support the earlier observations that lymphoma patients are at increased risk of acquiring lung cancer as compared to the general population [17]. This association has been described mainly in anti-lymphoma therapies, although the precise mechanism has not been elucidated yet. In 2 population-based studies on MF patients from the USA and Finland, Kantor et al. [3] and Väkevä et al. [18] have reported a 2.8- and 2.7-times higher risk of lung cancer, respectively [18]. Additionally, iatrogenic cancer risks in these populations were also considered. There is concern about a possible association between PUVA treatment and an increased risk of non-cutaneous cancer. According to Stern et al. the overall risk of non-cutaneous cancer was nearly identical to that expected in the general population [19]. In 4 out of 32 patients (3 men, 1 woman) lung cancer was diagnosed in our group. The diagnosis of co-existing cancer was preceded by MF in 3 cases. This association can be related to anti-lymphoma therapy. Although the precise mechanism has not been described, the influence of CTCL treatment to the skin microenvironment is suspected: Th1 to Th2 switch related to downregulation of STAT4 (because of IL-12 deficiency) and upregulation of STAT3 and STAT5 (induced by IL-2 and IL-15 as well as by cytokine independent JAK1/JAK3 signalling). Constitutive STAT3 and STAT5 activation in late stages increases survival and resistance to apoptosis and promotes Th2 and Th17 phenotypes, which is not only important in CTCL progression. CTCL treatment can also influence staphylococcal colonization – and it is well known that peptides produced by staphylococci can also promote the Th1 to Th2 shift. It is also known that tumour microenvironment macrophages (TAMs) contribute the immune suppressive environment and promote tumour growth not only in CTCL [20]. Three of 4 patients were treated systemically in our group: 2 with methotrexate and 1 with bexarotene and interferon α.
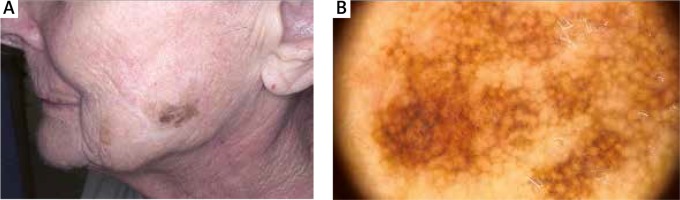
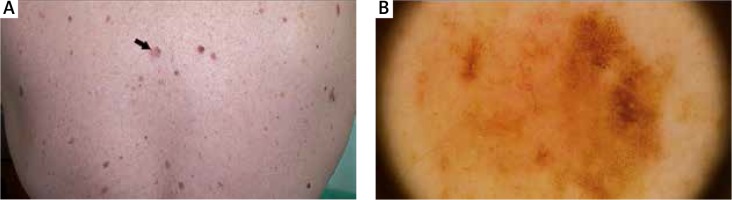
An elevated rate of malignant melanoma (MM) in patients with MF was also previously noted [21]. In our study, 2 patients were diagnosed with MM after the diagnosis of MF (Figures 4, 5). However, those patients had not received potentially carcinogenic therapy for their MF before the development of MM. The reasons for this association are unclear, but it may involve several factors related to the host or the environment. UV exposure is considered to be a major cause of cutaneous immunosuppression, and it is implicated as an aetiological factor of both melanoma and non-Hodgkin’s lymphoma [22, 23]. None of our patients with MF and MM was exposed to phototherapy before the diagnosis. But patients with MF and HL were exposed to phototherapy before the HL diagnosis. UV-specific p53 mutations have been detected in MF, particularly in tumour-stage disease [24]. Abnormalities in the p16 gene are also known to be found in MM and CTCL as well [25, 26].
Conclusions
To the best of our knowledge, our study is the first report of the incidence of other primary cancers in Polish patients with cutaneous lymphoma. However, this study has some limitations, such as a small number of cases, along with being a single-centre based study. More patients are needed to shed further light on the association between CTCL and other primary cancers. Generally, for the patients with CTCL, a careful follow-up examination is required to detect other cancers as soon as possible.