Introduction
Lichen planus (LP) is a chronic autoimmune inflammatory disease affecting the skin, nails, scalp, genital and oral mucosa. Oral lichen planus (OLP) affects up to 2.2% of the population, mostly middle-aged people, with a higher prevalence in women. Up to 60% of patients with cutaneous LP develop oral lesions, but only about 15% of patients with OLP present skin involvement [1–3]. The disease aetiology remains unknown; however, T-cell triggered apoptosis of oral epithelium basal cell keratinocytes is involved in the pathogenesis [1, 4]. Numerous factors such as stress, dental materials, infectious agents (e.g., hepatitis C virus), drugs, autoimmunity, diabetes mellitus and hypertension have been suggested as triggers for the lesions [2, 4, 5]. Oral manifestations of LP may present as papular, reticular, erosive, atrophic bullous lesions or plaque-like lesions usually involving bilaterally buccal, lingual and gingival mucosa [3, 5]. As no causative treatment of OLP is available, the management of the disease includes the elimination of predisposing factors, the use of topical corticosteroids, immunomodulators, retinoids, and the application of laser therapy [1, 3, 5, 6].
Oral lichenoid lesions (OLL) clinically and histologically resemble OLP but have identifiable aetiology and appear unilaterally. The most common factors associated with OLL are exposures to dental materials (especially amalgam), drugs (non-steroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors) and graft-versus-host disease (GVHD) [5, 7–9]. OLL triggered by direct contact with restorative materials is considered to be a delayed contact hypersensitivity reaction (type IV according to Gell and Coombs’ classification). To distinguish OLP from OLL, skin patch testing may be used as an additional tool in the diagnosis of atypical and treatment-resistant OLP [10–12].
Patch testing (PT) is a standard procedure used to diagnose contact allergy resulting from type IV hypersensitivity. It was first introduced at the end of the 19th century and Josef Jadassohn as well as Bruno Bloch are considered as pioneers, hence the reference to PT as the Jadassohn-Bloch technique. PT is performed by applying potential allergens under occlusion on the skin under standardized conditions. Test substances are prepared in various suitable vehicles such as petrolatum, water, hydrophilic gel, or solvents. The patch tests are applied to the upper back of the patient for 48 h. The reactions are checked initially after 48 and then within 72–96 h. According to the recommendations of the European Society of Contact Dermatitis, PT should be performed in patients presenting with contact dermatitis (including dermatitis related to occupation), but also mucous membrane eruptions in which delayed-type hypersensitivity is suspected [13].
Aim
This study was designed to investigate the prevalence of delayed hypersensitivity reactions in patients with OLP and identify the most common allergens that may exacerbate the disease.
Material and methods
Twenty patients diagnosed with OLP and undergoing treatment in the Gerodontology and Oral Pathology Department of the Poznan University of Medical Sciences were enrolled in this study. Subjects were referred to the above Department by their dentists or general practitioners when they presented with white non-removable lesions or/and burning mouth sensation. Patients with lesions which were due to contact with amalgam restorations, and who did not sign the informed consent form were excluded.
A detailed oral examination consisting of anamnesis and clinical evaluation was performed on all recruited subjects. The patients’ history included age, gender, subjective complaints related to the oral cavity, dental hygiene habits, addictions, and use of prosthetic appliances. Oral cavity examinations were performed in artificial light with a dental mirror by a qualified dental specialist. All patients underwent skin examination performed by a dermatologist in the Dermatology Department of the Poznan University of Medical Sciences. PT included the Polish Baseline Series and Dental Screening Series, the list of test substances is presented in Tables 1–3. PT were performed by applying haptens into Finn Chambers which were then mounted onto the normal skin of the back for 48 h. The PT reading was performed after 48 and 72 h, in accordance with the International Contact Dermatitis Research Group recommendations (Tables 1–3).
Table 1
Test substances used in the Polish Baseline Series until February 2020
Table 2
Test substances used in the Polish Baseline Series since February 2020
Table 3
Test substances used in the Dental Screening Series
Results
The study group comprised 18 women and two men with an average age of 63 years (range: 42–88, SD 11.53 years). The mean duration of the disease was 3 years and 9 months (range: 0.5–21 years). Eight (40%) patients presented reticular OLP, an equal number of patients suffered from atrophic/erosive OLP, in 3 (15%) cases, the co-occurrence of atrophic/erosive and desquamative gingivitis was noticed, and only 1 woman had a plaque-like type OLP. Fourteen (70%) subjects used denture appliances, and only 2 (10%) smoked tobacco. Thirteen (65%) patients reported subjective complaints such as burning sensation and pain. Half of the studied population presented positive reactions to the patch tests. A total of 18 contact sensitization reactions were confirmed in 10 female patients. Table 4 shows the distribution of positive PT reactions according to the clinical type of OLP (Figure 1).
Table 4
The distribution of positive PT reactions according to the clinical type of OLP
| Variable | N (%) | Patch test positive reactions n (%) |
|---|---|---|
| Total | 20 (100) | 10 (50) |
| Reticular | 8 (40) | 5 (62.5) |
| Atrophic/erosive | 8 (40) | 3 (37.5) |
| Atrophic/erosive + desquamative gingivitis | 3 (15) | 2 (66.7) |
| Plaque-like | 1 (5) | 0 |
Figure 1
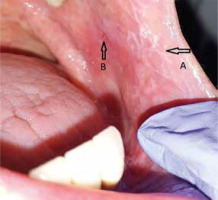
The white striae (A) and an erosion (B) on the buccal mucosa in a 72-year-old female patient allergic to the mercury
There were only 4 positive patch test reactions after 48 h. During the skin assessment, 72 h after the test application, an additional 14 positive responses were found. The results of positive reactions after 48 and 72 h are presented in Table 5.
Table 5
Positive PT reactions after 48 and 72 h
Some participants displayed more than one positive patch test reaction. The most frequently identified contact allergens in patients with positive patch test reactions were nickel (5) fragrance mix I (2) and gold (2).
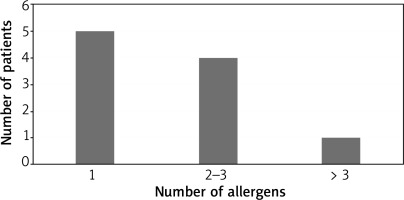
Five patients had a positive reaction to only one test substance, four to 2–3 allergens, and only one to more than three allergens (Figure 2).
Discussion
The clinical manifestations of contact allergy in oral and perioral diseases may present a broad spectrum of symptoms including cheilitis, stomatitis, perioral dermatitis, lichenoid lesions and even burning mouth syndrome [9, 14]. In this study, 50% of the examined patients had at least one positive reaction in PT, which is in agreement with studies performed by Yiannias et al. and Issa et al. [15, 16]. An even higher rate of positive responses to contact allergens in patients with OLP, namely 75% was presented by Kim et al. [17].
Many previous studies documented a strong association of OLL with amalgam restorations. The incidence of positive patch test reactions to amalgam compounds in several studies varied from 9.9% up to 65% and depended on the direct contact of the affected oral mucosa with the restorations [12, 14, 16, 18–20]. In patients with OLL, the replacement of amalgam fillings led to the resolution of the mucosal lesions. The improvement also depended on the topographical relationship between lesions and restorations. Lesions in complete contact with amalgam healed in 63% to 97% of cases, whereas in the case of partial contact, only about 27% of patients benefitted [12, 16, 18]. However, it was ineffective in patients with OLP due to the disease’s autoimmune background [15, 16, 18–20]. Moreover, Laeijendecker et al. found no positive patch test reactions to mercury in patients with concomitant cutaneous LP and Dunsche et al. reported a weak effect of amalgam replacement in a group with concomitant cutaneous LP [12, 18]. In our study, only 1 patient retained a positive reaction to mercury, despite the removal of amalgam many years before. On the other hand, she showed no positive response to any other applied allergens, including other amalgam metals such as copper or tin. The low rate of positive amalgam patch response may result from the inclusion of only OLP patients in our study. Likewise, Kim et al. and Raap et al. reported a low incidence of delayed mercury hypersensitivity in the OLP population [17, 21].
Nickel in stainless steel is one of the most common contact allergens worldwide. It is widely utilized in the household environment, e.g. in jewellery, watches, keys, clothing and kitchen accessories. Delayed type of hypersensitivity to nickel is found in 13% of the general adult population, and 8.6% of patients with contact dermatitis react positively to this allergen [22–24]. A retrospective analysis conducted in the Dermatology Department of the Poznan University of Medical Sciences showed a 56% nickel hypersensitivity reaction among patients with positive patch test results, corresponding to 18% of the studied population [25]. Similar outcomes were described in the studies of Polish population groups in Lodz (24 %) and Bialystok (21%) [26, 27]. A recent multicentre study conducted in six European clinics revealed nickel sensitization in 25% of the studied population (n = 906) and excluded the association of OLL with nickel and palladium sensitization [28]. Our results which showed a positive patch response in 50% of the test group and 25% of the studied population confirmed those observations. In dentistry, nickel alloys (Ni-Cr) are widely used in fixed prosthodontic appliances, metal and porcelain-fused-to-metal crowns and bridges, especially in developing countries [29]. Ahlgren et al. reported a similar rate of nickel hypersensitivity in their study, although this metal is not used in Sweden, except for orthodontics [19]. Likewise, Kim et al. and Rai et al. described a similar positive reaction to nickel sulfate and also to potassium dichromate [14, 17]. On the other hand, we failed to confirm any allergic reaction to chromium both as the metal and as metal ions.
Gold is another frequently identified metal allergen affecting dental patients with positive patch test reactions. Marell et al. reported the hypersensitivity to gold sodium thiosulphate in 50% of OLL patients with contact hypersensitivity (19.3% of the whole OLL subgroup) and 17% of OLP patients with contact hypersensitivity (8% of the entire OLP subgroup), which is in agreement with Khamayasi’ and our results [20, 30]. A slightly more frequent reaction among OLP patients was described by Kim et al. (33% of the whole OLP population and 44% of those with contact allergy) and Raap et al. (22% of OLP population) [17, 21]. In other studies conducted on subjects with OLL, the positive patch test response to gold ranged from 3.3% up to 29% of the subjects [30–35].
Fragrances, as ingredients of cosmetics, are considered one of the most common contact allergens worldwide [36]. They are also found in medication, food flavourings, household and washing detergents. Studies conducted in various Polish centres revealed contact hypersensitivity to fragrance mixtures in 8.3–20.4% of subjects, which is consistent with our results [25, 27, 37]. Larsen et al. reported positive PT reactions among 14.3% of patients with OLP and OLL in line with Torgerson et al., who described fragrance contact allergy in 17.1% of patients with lichenoid lesions and subjects with cheilitis and burning mouth syndrome [34, 38]. On the other hand, this was not supported by Budimir et al., who observed fragrance hypersensitivity only in patients with perioral dermatitis [31]. Similarly, Polańska et al. presented a case report of a 66-year-old female with papular and erythematous lesions in the upper and lower lip regions, who had both allergic and toxic reactions to fragrances (fragrance mix I and II and Lyral) [39].
Acrylates and methacrylates polymerize in ultraviolet light (or spontaneously) and are widely used in the production of plastics, textiles, glass fibres and artificial nails. Before the popularization of artificial nails, contact allergy to acrylates was considered an occupational disease mainly affecting dental personnel and orthopaedic surgeons. In dentistry, acrylates are present in dentures, composite resins, bonding materials and glass-ionomers [40–45]. Ramos et al. reported 30.3% of positive patch test reactions attributable to methacrylate, and 10.8% of the positive responses were related to the use of the acrylic dentures [40]. Muttardi et al. found 2-hydroxyethyl methacrylate to be the most common allergen among methacrylates and cyanoacrylates in their study, similar to Ramos et al. and Aalto-Korte et al. [40–42]. In our study, only 1 patient had a positive reaction to 2-hydroxyethyl methacrylate among all methacrylates tested, which supports their findings.
Although the skin assessment is commonly conducted 48 and 72–96 h after PT application, some authors suggest the use of additional tests due to delayed reactions [13, 46, 47]. Allergens generally associated with late positive responses are nickel, gold, mercury, cobalt, chromium, and neomycin [48]. Laeijendecker et al. showed that 35% of positive patch test responses occurred after 5 to 18 days [12]. Ahlgren et al. reported 26 additional positive allergic reactions seen only on day 7, which was a 25.2% increase and was statistically significant [48]. This observation may explain the difference between positive allergic response after 48 and 72 h in our study, however it has to be emphasized that each patient is informed about the necessity of an additional consultation in case of the appearance of a new skin reaction within the testing area.
The main limitation of this study is a relatively small sample size due to time-consuming test assessments, and the multiple visits, which were very challenging for the patients during COVID-19 pandemic.
Conclusions
This study has presented a high rate of contact allergic reactions in patients with OLP. It appears to be the first study to report the association between OLP and contact allergy in the Polish population. Further investigations on a larger group with the introduction of additional tests on day seven are required to confirm the influence of delayed hypersensitivity reactions on patients with OLP exacerbation.