Introduction
Asthma, affecting ~358 million people worldwide, is a heterogeneous disease characterized by chronic inflammation and enhanced airway responsiveness [1, 2]. Allergic asthma is the most common phenotype that affects a substantial proportion of patients with asthma. The prevalence of allergic asthma in adults increased from 5.0% in 1996 to 7.3% in 2016 [3].
The self-reported data from the European Health Interview Survey indicated that 1 in 26 people live with asthma in the Slovak Republic (Slovakia) [4]. According to the National Register of Asthma in Slovakia, 34% of patients have had asthma for > 10 years and 70% reported symptoms throughout the year. Furthermore, 31% of patients were reported to have moderate or severe persistent asthma and 27% had seasonal asthma symptoms [5]. An increasing trend in the incidence of occupational asthma due to allergens was also reported in Slovakia during 1980–2016 [6]. Despite advances in treatment options for asthma, a significant proportion of patients remain inadequately controlled [2].
Immunoglobulin E (IgE) is considered to be a key mediator of allergic reactions, and it plays an important role in the pathogenesis of allergic asthma. Upon exposure to an allergen, an allergic inflammatory cascade triggers the production of allergen-induced IgE by B-cells. These allergen-specific IgE molecules bind to the high-affinity receptor (FcεRI) present on the surface of mast cells and basophils and elicit an immune response [7, 8]. Omalizumab, an anti-IgE monoclonal antibody, inhibits the binding of IgE to high-affinity receptors and prevents the development of IgE-mediated allergic disease [9]. It was approved by the European Medicines Agency (EMA) in 2005 as an add-on therapy to improve asthma control in patients with severe persistent allergic asthma [10].
The therapeutic benefits of add-on omalizumab have been demonstrated in previous placebo (PBO)-controlled clinical trials [11, 12] and real-world studies [13–16]. However, there are concerns that asthma subjects enrolled in clinical trials do not reflect the real-life populations seen in clinical practice, due to stringent inclusion and exclusion criteria [17]. Moreover, the findings from PBO-controlled trials often cannot be translated to the more heterogeneous patient populations encountered in routine clinical practice. Therefore, additional evaluations in real-world settings across different geographical locations are required to understand the overall benefits of omalizumab in patients with severe allergic asthma.
Aim
The eXpeRience registry was a post-marketing observational registry initiated in Europe, Canada, and Asia to evaluate outcomes of omalizumab in patients with uncontrolled persistent allergic asthma in real-world settings [18]. Nearly a quarter of patients with uncontrolled persistent allergic asthma were enrolled from Slovakia [18]. The aim of this subgroup analysis was to evaluate the treatment effectiveness and safety of add-on omalizumab therapy in Slovakian patients enrolled in the eXpeRience registry.
Material and methods
Study design and patients
The eXpeRience registry was a 2-year, international, multicenter, open-label, single-arm, post-marketing observational registry for the collection of data from patients (N = 943) with uncontrolled persistent allergic (IgE-mediated) asthma receiving omalizumab in “real-world” clinical practice in Europe, Canada, and Asia [18]. Patients commenced omalizumab within the 15 weeks prior to inclusion in the registry. The enrolment period was ~2.5 years with a follow-up of ≤ 2 years for each patient after initiation of omalizumab. Patients treated with omalizumab in the preceding 18 months were excluded from the study with the aim of evaluating only newly indicated patients. Data were entered in the registry at ~16 weeks and at 8, 12, 18, and 24 months after initiation of omalizumab. The detailed methodology has been described elsewhere [18]. The current study was conducted in accordance with the Declaration of Helsinki of 1975, as revised in 2000. The study protocol was approved by the Bratislavsky Samosprávny Kraj ethics committee (approval number: 101167/2009-HF). Physicians obtained patients’ informed consent as per local regulations.
Assessments
Physician’s Global Evaluation of Treatment Effectiveness (GETE) was used to assess response to omalizumab. It shows an overall clinical evaluation of asthma control at 16 weeks, based on all available information (patient interviews, physical examination, and review of patient notes and diary [if used]). Following the week-16 assessment, GETE was not collected for the duration of the registry. Patients with an “excellent” or “good” response were considered responders.
The number of clinically significant asthma exacerbations and severe clinically significant asthma exacerbations was recorded at 12 and 24 months. A clinically significant asthma exacerbation was defined as a worsening of asthma as judged clinically significant by the physician, which required treatment with rescue oral or intravenous (IV) corticosteroids [19]. A severe clinically significant asthma exacerbation was defined as a clinically significant asthma exacerbation (i.e. worsening of asthma requiring treatment with systemic corticosteroids) with a reduction in peak expiratory flow (PEF) to < 60% of the patient’s predicted or personal best [19].
Asthma control was measured using the Asthma Control Test (ACT). The ACT is a patient-reported 5-item questionnaire, each with a 5-point scale (1–5). The overall score for each patient was the total of the responses to each question, giving a scale score between 5 (poorly controlled asthma) and 25 (well controlled asthma). The minimal clinically important difference (MCID) improvement in ACT has been defined as ≥ 3 points [20].
Other assessments included asthma-related medical resource utilization (hospitalizations, emergency room visits, unscheduled visits, or interventions) and number of days missed from school/work, oral corticosteroid (OCS) use in patients requiring oral steroids as asthma maintenance therapy, daytime clinical symptoms, activity limitations or nocturnal symptoms or awakenings, and use of rescue medication at 12 and 24 months. Lung function was measured by forced expiratory volume in 1 s (FEV1) % predicted and PEF at 12 and 24 months.
Safety assessments
Frequency of serious adverse events (SAEs) was assessed throughout the treatment period. SAEs were defined as events that were fatal or life threatening, resulting in persistent or significant disability/incapacity, or requiring inpatient hospitalization, or prolongation of existing hospitalization. Information on SAEs, regardless of suspected causality, was collected and reported within 24 h of the event’s occurrence. All SAEs were followed until resolution.
Statistical analysis
Data from all patients were summarized with respect to demographic and baseline (BL) characteristics and for effectiveness and safety. Because this was a registry of observational data, there was no formal statistical hypothesis testing or sample size estimation. Descriptive statistics were expressed as means with standard deviations (SD) or as frequency with percentages. Patient subsets included intent-to-treat (ITT), per-protocol (PP), and safety populations. ITT consisted of all patients enrolled in the registry, who received at least one dose of omalizumab and had at least one post-BL efficacy assessment. The PP population was based on the ITT population and excluded all patients with a major protocol deviation. The safety population consisted of all patients enrolled in the registry, who received at least one dose of omalizumab and had at least one post-BL safety assessment.
Results
Patients’ disposition, demographics, and clinical characteristics
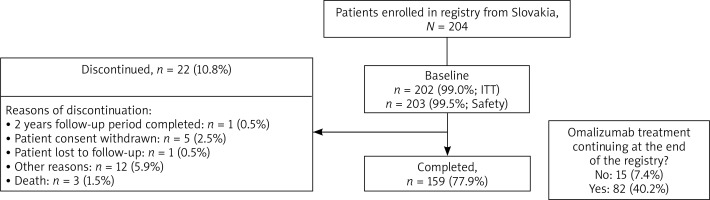
In total, 943 patients were enrolled in the eXpeRience registry; 204 patients were from Slovakia. Of these, 159 (77.9%) completed the 2-year follow-up, corresponding to 22.9% of all patients who completed the eXpeRience registry (Figure 1). Overall, 202 (99.0%) patients were included in the ITT population, 53 (26.0%) in the PP population, and 203 (99.5%) in the safety population.
The mean age of patients from Slovakia was 49.4 years. The majority of patients (> 80%) had uncontrolled asthma with daytime and nocturnal symptoms, and reduced lung function (FEV1 < 80% predicted). Patients’ demographics and BL clinical characteristics are presented in Table 1.
Table 1
Baseline demographics and clinical characteristics (safety population)
| Characteristic | Safety set (N = 203) |
|---|---|
| Age groups [years], n (%): | |
| 18–64 | 186 (91.6) |
| ≥ 65 | 17 (8.4) |
| Age [years] | 49.4 ±12.3 |
| Gender, n (%): | |
| Men | 73 (36.0) |
| Women | 130 (64.0) |
| Caucasian, n (%) | 201 (99.0) |
| Lung function (PEF or FEV1), n (%): | |
| < 80% predicted or personal best | 196 (96.6) |
| Exacerbations, n (%): | |
| None | 12 (5.9) |
| One or more/year (but not in the past week) | 155 (76.4) |
| One during the past week | 34 (16.7) |
| Patient’s current level of asthma control (investigator assessment), n (%): | |
| Partly controlled | 35 (17.2) |
| Uncontrolled | 168 (82.8) |
| Patient’s current level of asthma control (based on GINA report 2006 [19]), n (%): | |
| Partly controlled | 5 (2.5) |
| Uncontrolled | 197 (97.0) |
| Total number of clinically significant asthma exacerbations within the last 12 months* | 5.2 ±3.65 |
| Number of severe clinically significant asthma exacerbations within the last 12 months* | 2.6 ±2.19 |
| Number of asthma-related hospitalizations within the last 12 months* | 0.8 ±1.20 |
| Patient’s predicted FEV1 [l] | 2.9 ±0.74 |
| Patient’s FEV1 % predicted | 53.4 ±13.25 |
| PEF [l/min] | 222.9 ±92.76 |
| ACT overall score | 11.6 ±3.27 |
| ACQ overall score | 3.5 ±0.59 |
| mini-AQLQ overall score | 3.7 ±0.75 |
| Daytime symptoms, n (%) | 201 (99.0) |
| Limitations of activities, n (%) | 195 (96.1) |
| Nocturnal symptoms/awakening, n (%) | 195 (96.1) |
| Need for reliever/rescue treatment, n (%) | 201 (99.0) |
| OCS (maintenance monotherapy), n (%): | |
| Yes | 70 (34.5) |
| No | 133 (65.5) |
| Total daily dose (in prednisolone equivalent) [mg] | 16.9 ±10.81 |
| ICS maintenance mono- and combination therapy; combined total daily dose (in BDP equivalent) [μg] | 1720.1 ±773.14 |
| ICS (maintenance monotherapy), n (%): | |
| Yes | 46 (22.7) |
| No | 157 (77.3) |
| ICS (maintenance fixed-dose combination therapy [with LABA]), n (%): | |
| Yes | 172 (84.7) |
| No | 31 (15.3) |
ACQ – Asthma Control Questionnaire, ACT – Asthma Control Test, AQLQ – Asthma Quality of Life Questionnaire, BDP – beclomethasone dipropionate, FEV1 – forced expiratory volume in 1 s, GINA – Global Initiative for Asthma, ICS – inhaled corticosteroid, LABA – long-acting β2 agonist, OCS – oral corticosteroid, PEF – peak expiratory flow.
Physician’s Global Evaluation of Treatment Effectiveness
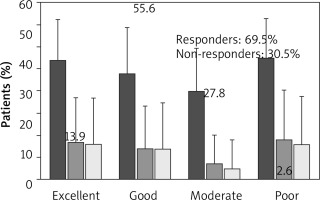
At week 16 (±1 week), 69.5% of patients were considered responders (13.9% had an excellent response and 55.6% had a good response), and 30.5% patients were non-responders (moderate response or poor response) (Figure 2). No patient reported ‘worsening of asthma’ as per physician’s GETE.
Exacerbations
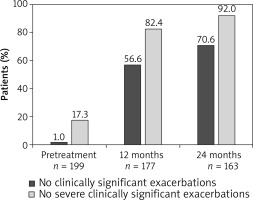
The mean annualized rate of clinically significant exacerbations in the ITT population was considerably lower at month 12 (mean ± SD: 1.0 ±1.69) and month 24 (0.6 ±1.12) compared with the pre-treatment period (5.2 ±3.64). Similarly, the annualized rate of severe clinically significant exacerbations decreased at month 12 (0.2 ±0.66) and month 24 (0.1 ±0.46) compared with the 12-month period prior to the start of omalizumab treatment (2.6 ±2.19). At months 12 and 24, a higher proportion of omalizumab treated patients reported no clinically significant or severe clinically significant exacerbations compared with the 12-month pre-treatment period (Figure 3).
Asthma symptoms and rescue medication use
The mean number of days that patients experienced daytime symptoms, activity limitations, or nocturnal symptoms or awakenings, and use of rescue medication reduced at month 12 and 24 compared with the BL (Supplementary Figure S1).
Lung function
Omalizumab improved the mean change in FEV1 (% predicted) from BL (mean ± SD) by 14.2 ±18.91 and 12.2 ±19.73 at months 12 and 24, respectively (Table 2). In the overall population, an increase of 9.8% and 8.7% in FEV1 (% predicted) was observed from BL at month 12 and month 24, respectively. PEF increased from BL (mean ± SD) by 61.5 ±117.63 l/min at month 12 and by 66.1 ±126.67 l/min at month 24 after omalizumab treatment. In the overall population, PEF increased from BL by 40.4 ±116.2 l/min at month 12 and by 34.0 ±132.2 l/min at month 24 [18].
Table 2
Change from baseline in lung function and asthma control after omalizumab treatment at 12 and 24 months (ITT population)
Asthma control
The overall score (mean ±SD) of ACT increased from 11.6 ±3.28 at BL to 18.9 ±3.96 and 20.3 ±3.81 at months 12 and 24, respectively. The MCID improvement in ACT (an increase of > 3 points vs. BL) was observed at months 12 and 24 (change in score 7.0 ±4.55 and 8.3 ±4.54, respectively) (Table 2).
Healthcare utilization and missed work/school days
Healthcare resource utilization and absenteeism from work/school reduced at months 12 and 24 with omalizumab compared with BL. The asthma-related medical healthcare uses (mean ± SD) were 0.3 ±0.83 at month 12 and 0.3 ±0.82 at month 24, lower than at BL (7.7 ±5.96) (Supplementary Table S1).
Oral corticosteroid use
At BL, the maintenance therapy consisted of OCS monotherapy (28.4%), ICS monotherapy (26.5%), LABA monotherapy (16.4%), ICS/LABA fixed-dose combination therapy (82.1%), short-acting β2-agonist (SABA) monotherapy (5.1%), LAMA monotherapy (20.3%), SABA/LAMA fixed-dose combination therapy (3.4%), LT4 inhibitors (61.4%) and other monotherapies (26.5%) [18].
The proportion of patients on OCS as maintenance therapy reduced to 17.0% at month 12 and 15.3% at month 24 compared with BL (34.7%) (Supplementary Table S2). In the overall population, 28.6% patients were on maintenance OCS therapy at BL, which reduced to 16.1% and 14.2% at 12 and 24 months, respectively [18]. Reduction in OCS dose or discontinuation of OCS use was observed in 63.9% and 70.4% of patients at months 12 and 24, respectively.
Safety
In total, 8 (3.9%) patients reported 16 SAEs, 13 (81.3%) of which were not suspected to be related to omalizumab treatment (Table 3). Omalizumab was permanently discontinued in 11 (68.8%) patients, dosage was temporarily interrupted in 3 (18.8%) patients, while no change in dosage occurred in 2 (12.5%) patients. SAEs of special interest (anaphylaxis and thrombocytopaenia) were reported in 2 (1.0%) patients. There were 3 deaths, one each due to cholestatic jaundice, sepsis, and asthma. No death was related to the study drug as per the investigator’s judgment.
Table 3
Serious adverse events (SAEs) in patients receiving omalizumab therapy (safety population))
Discussion
The current subgroup analysis from the eXpeRience registry, comprising patients from Slovakia, showed that omalizumab was associated with marked improvements in GETE, lung function, and asthma control, while also reducing exacerbations, use of OCS and rescue medication, and healthcare utilization at the assessed time points. This is the first real-world study reporting the effectiveness of omalizumab in patients from Slovakia. Interestingly, of all the countries included in the eXpeRience registry, Slovakia had the maximum number of centres (184) and the highest number of patients (n = 204, 22.9%).
The response to omalizumab treatment as assessed using the physician’s GETE indicated that the majority of patients (~70%) achieved an excellent or good response at week 16, consistent with the results seen in the overall population of the eXpeRience registry [18]. These results were further supported by QUALITX study, in which nearly 74.6% of patients reported excellent/good efficacy to omalizumab at week 20 [21]. In contrast, other studies have reported a higher ratio of omalizumab responders (~90–96% [PROXIMA] [15]; 78.8% [XCLUSIVE] [22]; and 89.2% [23]). Our results were not surprising because most patients in the Slovakian subpopulation had uncontrolled asthma (82.8% per investigator assessment and 97.0% per GINA report 2006) and worst asthma control (5.2 exacerbations/year) at study initiation, which could have led to a lower GETE score.
Uncontrolled persistent allergic asthma is often associated with frequent exacerbations that pose a significant burden to patients [24]. In the present study, omalizumab treatment showed a decrease in the mean annualized rate of clinically significant asthma exacerbations from 5.2 to 0.6 during a 24-month period. Moreover, the proportion of patients with no clinically significant exacerbations increased to 70.6% following 24 months of omalizumab treatment. These findings are largely consistent with those reported by Braunstahl et al. for the overall population of the eXpeRience registry [18], which was further complemented by multiple studies [13, 15, 22, 25, 26]. The proportion of patients with no or reduced asthma exacerbations ranged from 53.0 to 62.4% in previous studies conducted for 6 months to 24 months in different clinical settings [27–29].
Patients with uncontrolled allergic asthma show a decline in lung function, and many clinical trials and real-life studies have demonstrated improvements following treatment with omalizumab [11, 12, 15]. In our study, an improvement in FEV1 (% predicted) and mean PEF was observed at month 12; this remained consistent at month 24, indicating the long-term benefit of using omalizumab. It is worth noting that Slovakian patients had worse lung function at BL compared with other European populations; hence, a marked improvement in FEV1 was observed with omalizumab at 12 and 24 months. The APEX study demonstrated improvement in FEV1 (% predicted) (69.9 at BL to 78.6 at month 12), PEF (l/min) (299.2 at BL to 326.8 at month 12) after omalizumab treatment [13]. Compared with APEX, the current study observed greater improvements in FEV1 and PEF, which could have been due to high disease severity at BL. In a Czech Republic subpopulation study of the eXpeRience registry, at month 12, treatment with omalizumab resulted in improvement in FEV1, which was demonstrated by a mean change of 273 mL from BL. Likewise, PEF (l/min) was also improved, with a mean change of 11.01 from BL to 32.82 at month 12 [30].
In the current study, omalizumab improved asthma control, and the number of patients achieving asthma control stabilized after 16 weeks of treatment. A higher percentage of patients had controlled or partly controlled asthma in the overall population in the week prior to months 12 (85.0%) and 24 (87.1%) than was noted at BL (24.2%) [18]. In a retrospective study by Molimard et al., asthma control was lost in 34 (55.7%) patients with persistent allergic asthma after 13 months following discontinuation of omalizumab [31]. In the EXCELS study, the percentage of omalizumab-treated patients with well-controlled asthma (ACT score > 20) increased from 45% at BL to 61% at month 60. For patients who started omalizumab, the percentage with adequately controlled asthma increased to 51% at month 6 and to 60% at month 60 from 25% at BL [32]. This was further supported by a recent meta-analysis of observational studies, which has clearly demonstrated a significant improvement in ACT (mean difference [95% CI]: 4.44 [3.55-5.34]) following 16 weeks of omalizumab treatment [33].
A higher proportion of Slovakian patients were on maintenance OCS at BL (34.7%), indicating an uncontrolled nature of asthma in more than one-third of the population. Omalizumab was associated with a reduction in OCS use of 17.0% at month 12 and 15.3% at month 24, which is a potential reason for improved asthma control. In the overall population, the incidence of OCS maintenance use was lower at 24 months when compared with 12 months and BL (14.2% vs. 16.1% and 28.6%). The results are in line with previous real-world studies conducted in France [31, 34], Belgium [26], and the UK [13]. In the APEX study, the mean daily OCS dose decreased by 5.5 mg (25.6%; p < 0.001) in patients with severe persistent allergic asthma and 66 (48.5%) patients stopped OCS completely within 1 year of omalizumab initiation [13]. In a meta-analysis, the reduction in OCS use was estimated to be 32% (RR [95% CI]: 0.68 [0.57 ±0.82]; p < 0.01) with 16-week omalizumab treatment in patients with severe allergic asthma, compared with BL [33].
No new safety signals were observed in this study. Of the 64 (6.9%) patients with SAEs reported overall [18], 8 (3.9%) patients represented the Slovakian population. The majority of SAEs were not suspected to be related to omalizumab treatment (similarly to the overall study [18] and Czech Republic population [30]).
The international population with severe asthma tends to be more prevalent in Europe, with the Slovakian subpopulation from the eXpeRience registry being the worst (5.2 exacerbations per year before treatment), followed by the UK (5 exacerbations per year) and Italy (3.7 exacerbations per year) [35], when compared with American and Asian subpopulations. These results were in line with observations from the global eXpeRience registry and other global studies on omalizumab [16, 18, 36]. A potential limitation of this study is its inherent observational nature, which could have added an element of bias to the overall findings. Additionally, this study was not adequately powered for pairwise comparisons, and the results were based on descriptive analysis.
Conclusions
The results from this subgroup analysis of the eXpeRience registry for the Slovakian population complement the overall population and further support findings from randomized controlled trials [11, 12] that omalizumab is effective in achieving asthma control when added to current therapy in patients with uncontrolled persistent allergic asthma in real-life settings. Moreover, this study shows a tendency for favourable effects of omalizumab in patients with severe asthma; however, the study population was small.