Introduction
Atopic dermatitis (AD) is a chronic inflammatory disease characterized by eczematous lesions. The prevalence of AD is estimated to be almost 30% of paediatric population and up to 10% of adults with a threefold increase in industrialized countries [1]. Many studies have demonstrated the involvement of genetic factors, epidermal barrier dysfunction, immune dysregulation, emotional and environmental stimuli in its development and progression. Both the innate immune system and activation of T-cell mediated inflammatory pathway play central roles in disease development and progression. The acute Th2 response results in secretion of pro-inflammatory mediators such as interleukin (IL) 4 (IL-4), IL-13, IL-31, (TARC)/CCL17 and S100As [2]. As the diseases transit to the chronic phase the immune response is predominated by Th1 T-cell subsets. The role of Th22 and Th17 lymphocytes in both acute and chronic phase of the disease was noticed [3]. The interplay between Th22, synergistically with the Th17 cytokine IL17 and epidermal barrier, causes its disruption by suppression of major termination proteins such as filaggrin, loricrin or involucrin [4]. They also enhance keratinocyte migration and induce epidermal hyperplasia in both extrinsic and intrinsic AD [5, 6]. IL-17 appears to be implicated in secretion of many proinflammatory cytokines, antimicrobial molecules, chemokines and upregulating granulopoietic factors [7].
The IL-17 family of cytokines consists of six members (IL-17A-F). It has been reported that the levels of IL-17A and IL-17F in serum of both adults and infants are elevated, with limited Th1 polarization for the second group [8]. The IL-17A and IL-17F gene loci are adjacent to each other on the same chromosome 6p12 with a difference in the expression pattern [9, 10]. Both of them form homodimers and heterodimers with a higher activity to IL-17A and lower potency to IL-17A/F complex [11]. The biologic effects of these complexes differ. Homodimer IL-17A/A is mainly involved in host defence against pathogen infections (bacterial, fungal and parasitic) and autoimmune response, while homodimer IL-17F/F promotes inflammatory response and skin barrier maintenance [12]. The data about biologic characteristics of heterodimer IL-17A/F are still limited. Certainly, the heterodimer is similar in function to IL-17A and IL-17F. Therefore, it has been recently reported that IL-17A/F increases neutrophil and chemokine expression in the airways and stimulates the growth of vascular endothelial cells in physiological and pathological angiogenesis [13, 14].
The expression of these interleukins can be modified by polymorphisms in the IL-17 gene. Polymorphisms in IL-17 interleukins have been analysed in many inflammation-related diseases, autoimmune diseases and cancers [15–21]. The following were most commonly analysed: rheumatoid arthritis, psoriasis, ulcerative colitis, osteoarthritis, and asthma. The most investigated single nucleotide polymorphisms (SNPs) were rs2275913 IL-17A and rs763780 IL-17F.
Aim
The aim of this study was to determine the serum concentrations of IL-17A/F and additionally IL-17A, IL-17F, IL-13, IL-4 in patients with atopic dermatitis and the control group and to examine the association of IL-17A/F and rs2275913 IL17A and rs763780 IL-17F gene polymorphisms in those patients.
Material and methods
Subjects
We examined 30 patients with atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka and 30 healthy controls randomly recruited by advertising. Both of the groups were aged 2–12 years.
From January 2018 to October 2019 the subjects were recruited and observed in our Department of Dermatology, Paediatric and Oncological Dermatology in Bieganski Hospital of the Medical University.
Data collected in baseline evaluations included clinical data of each patient, including the symptoms, duration of disease, concomitant medications, atopic comorbidities (allergic rhinitis, allergic conjunctivitis, food allergy, asthma), personal and family medical history. In AD patients at the time of recruitment, the severity of the disease was assessed by collection questionnaires such as: Eczema Area and Severity Index (EASI), Investigator Global Assessment (IGA) and Scoring Atopic Dermatitis (SCORAD).
The present study was approved by the ethics committee of the Medical University of Lodz. All legal representatives of the participants were provided with information regarding the protocol in a simple language. The children were included in the study after written informed consent was obtained from the parents.
Exclusion criteria
Both patients with AD and the control group were excluded if they met the following criteria: (1) active infectious disease, (2) use of systemic and topic antibiotics 2 weeks prior to study enrolment, (3) use of systemic and topical glucocorticosteroids 2 weeks prior to study enrolment, or (4) use of immunosuppressive drugs.
Serum levels of IL-17A, IL-17F, IL-17A/F, IL-13 and IL-4
On the day of examination, two blood samples were collected from each participant from the ulnar vein into EDTA-containing test-tube and a tube without anticoagulants. Blood was drawn under fasting conditions. Promptly after drawing blood, the tubes without anticoagulants were very gently inverted 10 times. Biological material from patients collected in sterile clot tubes was centrifuged at room temperature for 20 min at 800xg. Samples were put in sterile Eppendorf tubes and stored frozen at–20°C until assessment.
The blood collected in the ethylene diamine tetra acetic acid (EDTA)-containing test tube was stored at –80°C until molecular investigations.
After collecting all serum samples from the test group and control group, the previously protected material was put on the microtiter plate for individual interleukin. Serum levels of IL-17A, IL-17F, IL-17A/F, IL-13, and IL-4 were measured using enzyme-linked immuno-sorbent assays (ELISA) (Diaclone SAS, 6 Rue Docteur Girod, Besançon, Cedex France) according to the manufacturer’s instructions.
The minimum detectable level of IL-17A, IL-17F, IL-17A/F, IL-13 and IL-4 is less than 2.3 pg/ml, 6 pg/ml, 6.6 pg/ml, 1.5 pg/ml and 0.7 pg/ml, respectively.
Isolation of genomic DNA
Genomic DNA was extracted from blood cells of participants using the Blood Mini genomic DNA purification kit (A&A Biotechnology, Gdynia, Poland) according to the manufacturer’s instructions.
Real-time quantitative PCR protocol by TaqMan MGB probe assay
Genotyping of IL-17A rs2275913; G>A and IL-17F; rs763780; T>C was performed by fluorescence real-time quantitative polymerase chain reaction (Real Time quantitative PCR). All samples were genotyped in duplicate using allelic discrimination assays with pre-validated TaqMan MGB dual-labelled probes, which were purchased from Eurogentec, Poland. TaqMan probe kits contained a pair of primers and two allele-specific probes designed to target the polymorphism. The design of sequence-specific oligonucleotide primers was based on reference sequences published in the National Center for Biotechnology (NCBI) GenBank.
TaqMan MGB probes had incorporated a 5’-end fluorescent reporter dye (such as FAM, HEX for IL-17A rs2275913 and Texas Red, Cy5 for IL-17F rs763780) and a 3’-end minor groove biner-nonfluorescent quencher (MGB). One 5’-end reporter dye was complementary to the wild-type SNP Allele (HEX was specific for IL-17A rs2275913 Allele G and Cy5 for IL-17F rs763780 Allele T) and the other to the mutated Allele (FAM corresponded to IL-17A rs2275913 Allele A and Texas Red Allele C for IL-17F rs763780).
Before the reaction, the probe did not emit fluorescence as it is absorbed by the quencher. The probe was complementary to the fragment of the sequence defined by the primers (5 ‘and 3’ or F – Forward and R – Reverse). The sequence of primers and probes are listed in Table 1.
Table 1
List of primer and probe sequences for TaqMan-MGB probe based real time PCR assays
Real-time PCR was implemented in the first tested reaction mixture consisting of 5 μl Nzy Speedy qPCR Probe MasterMix, 0.2 μl F-primer, 0.2 μl R-primer, 0.2 μl of each probe and 2.7 μl of water and then for each reaction 1.5 μl (25 ng) of prepared DNA samples with 8.5 μl mixture was added. Real-time PCR amplifications were performed in CFX96 Real-Time System (BIO-RAD) according to the following protocol: initial denaturation at 95°C for 7 min; amplification (45 cycles) at 95°C for 15 s; 60°C for 1 min.
As amplification proceeded, the Taq polymerase enzyme cleaved the bound probe and a fluorescent signal was generated in the channels for FAM and HEX or Texas Red and Cy5 depending on the polymorphism tested.
A fluorescent signal from only the FAM dye indicated homozygosity for Allele A; the presence of only HEX dye fluorescence showed homozygosity for Allele G and the presence of both fluorescent signals specified Allele A/G heterozygosity. The same sequences were observed for Texas Red and Cy5 fluorescent probes respectively for allele C and T.
The genotypes were analysed using CFX96 BIO-RAD software.
Statistical analysis
Categorical variables are presented as numbers with an appropriate percentage (case number/total number). The χ2 test with appropriate corrections applied depending on the size of the subgroups was used for their analysis. Continuous variables are presented as a median with the values of the lower and upper quartiles (25–75 percentile). The normal distribution was verified by the Shapiro-Wilk W test. The differences between the groups were assessed using the Mann-Whitney, Kruskal-Wallis test or the χ2 test (variables with a distribution other than normal). The analyses were done by using Statistica software (v.6.0 Statsoft, Tulsa, OK, USA).
Hardy-Weinberg equilibrium (HWE) test was used to include SNPs rs2275913 IL-17A and rs763780 IL-17F for investigation. Deviations from HW equilibrium were confirmed by manual recalculation.
When presenting the results, p-values were given and p-value < 0.05 indicated statistical significance.
Results
Demographic and clinical characteristics
Altogether, 30 subjects with AD and 30 controls, median age was 8 (25–75%: 4–10) participated in the study. There was a slight male preponderance in 60 patients with 51.67% of boys. There was a significant difference in allergic comorbidities between the subjects. The history of food allergy was more common in children with AD than in healthy children (40.00% vs. 10.00%, p = 0.0073).
The AD duration ranged from 5 days to 3 years. Its mean duration was 2.95 months. The median age of diagnosis was 5.9 months (minimum 0.49 months, maximum 9 years). The severity grading of the AD group was done according to SCORAD, IGA and EASI. The study of the AD population showed a median value of EASI score of 11.4 ±7.4, SCORAD 45.9 ±14.2 and IGA 3 ±0.54.
IL-17A/F, IL-17A, IL-17F, IL-4, IL-13 in serum
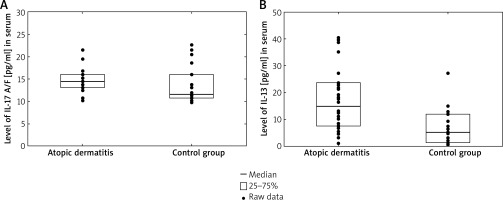
The serum levels of IL-17A/F in patients with AD (14.50 pg/ml, range: 13.13–16.02) were significantly higher than those in control subjects (11.60, range: 10.76–16.02, p = 0.025). On the contrary, there was no difference in serum IL-17A, IL-17F levels between patients with AD and control subjects. Additionally, we found a significantly higher level of IL-13 in AD patients compared with healthy individuals (3.39 pg/ml, 25–75%: 1.95–4.78 vs. 5.32 pg/ml, 25–75%: 1.47–12.12, p = 0.0019) (Figure 1). Our study revealed no statistically significance in the level of IL-4 in children with AD versus the control group.
Figure 1
Serum IL-17A/F levels (A) and IL-13 levels (B) in atopic dermatitis group were higher than controls (3.39 pg/ml 25–75%: 1.95–4.78 vs. 5.32 pg/ml 25–75%: 1.47–12.12, p = 0.0019 and 3.39 pg/ml 25–75%: 1.95–4.78 vs. 5.32 pg/ml 25–75%: 1.47–12.12, p = 0.0019, respectively)
The dermographic parameters, like sex and age were not correlated with the concentration of interleukins.
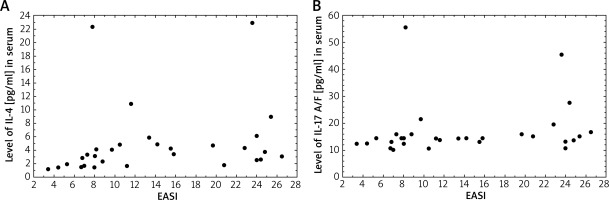
Correlations between the serum level of interleukins and SCORAD and IGA severity assessments were evaluated in patients with AD. Among 2 types of scales, serum IL-13 and IL-4 levels were moderately strongly associated with SCORAD scores in patients with AD (R = 0.37; p = 0.045 and R = 0.44; p = 0.015, respectively). On the other hand, our results demonstrated a strong correlation between EASI score and IL-17 A/F and IL-4 serum levels (R = 0.48; p = 0.037 and R = 0.48; p = 0.0077, respectively) (Figure 2). There was a difference in the mean serum IL-17 A/F levels between the investigated groups with medium disease activity (p < 0.005).
Figure 2
Correlation between serum IL-4 levels (pg/ml) (A) and IL-17A/F levels (pg/ml) (B) and EASL (R = 0.48, p = 0.0077 and R = 0.48, p = 0.037, respectively)
These results supported a positive relationship between duration of AD and serum concentration of IL-13 (R = 0.38; p = 0.038. On the contrary, a negative, moderately strong, statistically significant association was observed between the duration of AD and the concentration of IL-17 A/F (R = –0.40; p = 0.030).
IL-17A and IL-17F alleles and genotypes in AD patients and healthy controls
All observed allelic distributions of the study populations were in the normal range within Hardy-Weinberg equilibrium (HWE).
The distribution of the IL-17F rs763780 and IL-17A rs2275913 genotypes among AD subjects and the control group is shown in Table 2.
Table 2
Distribution of the IL-17A, IL-17F alleles and genotypes in Polish patients with AD and healthy individuals
| Variable | Patients | Controls | ||
|---|---|---|---|---|
| N | % | N | % | |
| IL-17A: | ||||
| AA | 3 | 43 | 4 | 57 |
| AG | 18 | 58 | 13 | 42 |
| GG | 9 | 41 | 13 | 59 |
| G | 27 | 51 | 26 | 49 |
| A | 21 | 55 | 17 | 45 |
| IL-17F: | ||||
| CC | 0 | 0 | 0 | 0 |
| CT | 3 | 60 | 2 | 40 |
| TT | 27 | 49 | 28 | 51 |
| C | 3 | 60 | 2 | 40 |
| T | 30 | 50 | 30 | 50 |
The frequency of IL-17A (rs2275913) A and G alleles in the total group of AD patients did not differ significantly as compared to the control group (OR = 1.73, 95% CI: 0.61–4.84, p = 0.4348 and OR = 0.43, 95% CI: 0.15–1.24, p = 0.1872, respectively).
Also, the frequency of IL-17F (rs763780) C and T alleles in the total group of AD patients did not reach statistical difference as compared to the control group (OR = 1.55, 95% CI: 0.24–10.05, p = 1.00 and OR = 0.64, 95% CI: 0.10–4.15, p = 1.00, respectively).
Additionally we analysed the association between IL-17A and IL-17F polymorphisms and the serum concentrations of IL-17A and IL-17F. We did not detect any association between the studied polymorphisms and cytokine IL-17A or IL-17F.
None of the analysed polymorphisms was found to be associated with age of patients, disease duration, disease severity or progression.
Discussion
The understanding of the role of cytokines in modulation of immune response in atopic dermatitis is crucial. The major Th2-cell response in the acute phase is caused by cytokines such as IL-4, IL-13, IL-5 and IL-31, which increase the epidermal defect in AD skin. Among those cytokines, our findings showed increased levels of IL-13 in serum of AD patients, which correlated with the severity of disease. Recent studies suggested a growing body of evidence regarding IL-13 as a major cytokine in Th2 robust [22]. The overexpression of IL-13 in skin in comparison to IL-4, which was undetectable or found at a low concentration, gave an assumption of a significant role in the epidermal barrier function, recruitment of inflammatory cells and skin dysbiosis. The other cytokines recruited in epidermal response, which are among others upregulating antimicrobial peptides are IL-22 and IL-17 [23, 24]. The upregulation of IL-17 is noticed in many skin diseases such as psoriasis, systemic sclerosis and mycosis fungoides in both sera and skin lesions [25, 26]. It was discovered that IL-17 was significantly increased in AD skin in contrast to non-lesional skin but it was lower than in psoriatic plaque [27, 28]. The literature shows differences between IL-17 concentration in skin lesions depending on the ethnicity and the age of AD patients. Recent studies suggested that in Asian population and in children population, IL-17/IL-22 expression in skin biopsy was significantly higher in comparison to European and American patients with AD [29]. The investigations demonstrated a higher concentration of the interleukins in acute lesions (defined as erythematous AD lesions less than 4 days from onset) rather than in chronic lesions [30, 31]. Certainly, acute lesions are characterized by upregulation mainly by Th2 T-cell subsets (IL-4, IL-13, and IL-31) and IL-22. The transformation to the chronic course of the disease is due to intensification of Th17/Th22 and Th2 immune axis and activation of Th1 immune response. In comparison to several IL-17-regulated products detected in acute and chronic skin lesions, the IL-17 cytokine expression was less significant. Moreover, the data concerning the meaning of IL-17 in serum are still inconclusive. In Qi Tan et al. study the serum level of IL-17 was elevated as compared to the control group but it was not statistically significant. On the contrary, Koga et al. showed detectable IL-17 both in serum and skin lesions in AD patients [32, 33]. Our findings of increased IL-17A/F levels in the serum of AD patients in comparison to the healthy group supports the significance of Th17cells in AD. The concentration of IL17 A/F was associated with severity of AD in IGA and EASI scales (median for all AD patients was moderate: IGA 3 and EASI 11.3). The negative correlation between IL-17A/F and duration of AD disease was statistically significant (R = –0.40; p = 0.030), which indicated Th17 polarization at the early stage of the disease in paediatric patients. This may suggest an important role of IL-17A/F in shifting from the acute to the chronic phase of the disease. Interestingly, the present study found a detectable level of IL-17F and IL-17A in AD patients but it was not statistically significant. Few studies reported an association of IL-17A and IL-17F and SCORAD in Asian population [34, 35]. More conflicting and scarce results are found in the association of IL-17 A and IL-17F gene variations in AD. When the IL-17 rs763780 in Holster et al. study was determined in 187 subjects with atopic disease, the children age 11-13 were at increased risk of atopic dermatitis [36]. Our group consisted of patients at the age of 4–10 years, which was confirmed in Holster et al. study not to be associated with IL-17 rs763780 SNP. In another study, the same genotype variant was searched in 160 adults with AD documenting no correlations. Numerous studies showed that the presence of IL-17F rs763780 genotype is noticed mainly in asthma, considering an increased IL-17F level gene expression in the bronchoalveolar fluid in asthmatic lung [37].
IL-17A has a similar role in promotion of inflammatory cells in the airway mucosa. In a meta-analysis of 5016 subjects, allele G of IL-17A rs2275913 polymorphism has been revealed as a protective factor for occurrence of asthma in children, but not in adults [38]. The link between IL-17A SNP and other atopic diseases such as allergic rhinitis, conjunctivitis and atopic eczema is of limited evidence in the literature. The presence of A/A genotype in IL-17A rs2275913 has been reported in severe AD patients but with the coexistence of asthma [39]. The study revealed a high trend towards A/A, G/A genotypes and A allele in 166 AD subjects with no relationship with an increased AD risk. Likewise, we found a higher frequency of G/A genotype (IL-17A rs2275913 polymorphism) in our study, although without an increased risk of AD development. Additionally, a Chinese study offered evidence of variation in IL-17-G152A to predispose early-onset AD, more possibly with concomitant asthma, with a higher Th17 cell percentage, Th17 mRNA level and IL-17 serum concentration in A/A and G/A genotypes. The presence of certain genotypes of IL-17 in the studies may impact on protein translation and expression of IL-17 in tissue or serum. We suspect that the polymorphic variants can influence patients’ phenotype altering the course of the disease. Our study evaluated only 60 children and two IL-17 SNPs, which is a certain limitation. Other variants of IL-17 and a larger size of population with a wide spectrum of epigenetic and environmental factors need to be further studied for precise phenotype specification.
Our AD patients showed a detectable concentration of IL-17 but in a form of heterodimers. Studies in vivo revealed that this a two-face cytokine [40]. Activation of CD4+ T cell population mediated IL-17F at 10-fold higher levels than IL-17A, explaining that the most of the IL-17A cytokine occurs in the form of IL-17A/F heterodimer. The biological function of this heterodimer includes all of the properties of both IL-17A and IL-17F including an increase in IL-6 and CXCL1 signalling. Wright et al. suggested that heterodimers are even more potent than IL-17A in enhancing chemokine expression [41]. The signalling of all three cytokines need a receptor complex consisting of IL-17RA and IL-17RC [40]. In comparison to IL-17A and IL-17F, IL-17A/F is assumed to form two separate signalling complexes with IL-17RA and IL-17RC, which can open a new target for the biologics pathway. Currently the only registered biologic approved for both adults and adolescents with AD is an antibody directed against the IL-4/IL-13 receptor (dupilumab) [42]. Great progress has been made with JAK inhibitors or biologics against IL-13 or IL-31 [43]. However, the investigations concerning therapy targeting the components of IL-17 pathway is still at the clinical trial level. Fezakinumab, anti-IL22 blocker which has synergistic properties to IL-17 as a good treatment response, while ustekinumab blocking anti-IL-12/23 p40, which is required for Th17 cell development, did not meet the expectations in treatment of AD [44, 45]. Additionally, the preliminary results of phase 2 clinical trials of anti-IL-17A (secukinumab) and anti-IL-17C (MOR106) molecules did not show significant efficacy in a small study group [46, 47]. Yet, no studies have been conducted on IL-17 A/F, IL-17RA and IL-17RC directed therapy or blocking both IL-17A and IL-17F isoforms (bimekizumab). Additional research concerning these treatment options with a large sample size group, targeting patients with increased Th17 activation (paediatric with Asian ethnicity and intrinsic AD) could be an interesting approach for AD.
Conclusions
Our study demonstrated that the serum IL-17A/F levels in atopic patients are higher than those of the control group, positively correlating with the severity of the disease. We have also noted a striking increase in IL-17 A/F concentration in parallel to the early phase of the disease in paediatric patients. As a result, the meaning/significance of IL-17 A/F in serum is a distinctive interleukin in AD pathogenesis. These findings may provide new insight into the development of novel therapeutic strategies for AD patients.
However, IL-17A and IL-17F in sera of AD patients and their gene variants (rs2275913 IL17A and rs763780 IL17F) were not associated with an elevated risk to develop the disease. Further variants in IL17A/F gene in larger number of studied group should be investigated to determine their role in etiology of AD.








