Introduction
Hepatocellular carcinoma (HCC) is the sixth commonest solid tumor globally and the third reason for cancer-related mortality [1]. Chronic hepatitis C virus (HCV) infection poses a big risk for HCC [2]. In Egypt alone 25,399 new cases of HCC were discovered during 2018 according to the World Health Organization (WHO) registry. This is mainly due to the high burden of HCV infection [3].
Introduction of directly acting antiviral agents (DAAs) has resulted in higher chances for HCV elimination [4]. Despite that, the risk of HCC occurrence is still present [5].
Before the era of DAAs the annual risk of developing HCC among patients with HCV related cirrhosis was around 2-8% [6]. Many studies have suggested that DAA exposure increases the risk of de novo occurrence of HCC while other studies suggested that achieving a sustained virologic response (SVR) decreases that risk [7].
Tolloid-like 1 gene (TLL1) variant rs17047200, located on the short arm of chromosome 4 region 12, has been reported to be associated with HCC development among the Japanese population. TLL1 rs17047200 AT/TT was found to be an independent risk factor for HCC development after HCV treatment [8].
Identifying persons at higher risk of developing HCC is of utmost importance [9]. Our aim was to identify different predictors of de novo HCC occurrence among cirrhotic patients after initiating HCV treatment using DAAs, and to assess different available scores, such as the FIB-4, ALBI and PALBI scores, aiming at better patient stratification.
Material and methods
Materials
From 1st October 2018 to the end of July 2019, 1382 subjects with positive antibodies to HCV sought treatment for HCV at Alexandria University Viral Hepatitis Treatment Unit. On performing PCR, only 1172 showed viremia. 110 patients did not receive treatment due to presence of medical contraindications according to the Egyptian National Treatment Program of Hepatitis C guidelines [10] such as pregnancy, lactation, Child-Pugh class C and discovery of untreatable HCC or other malignancies. Out of the remaining 1062 patients, 547 patients had liver cirrhosis and initiated DAA treatment for HCV at our unit. Cirrhotic patients were identified as either those with clinically evident cirrhosis or those with pretreatment FIB-4 > 1.45 whose pretreatment acoustic radiation force impulse (ARFI) is 2.19 m/s or more [11, 12].
One patient was excluded from this study due to previous history of HCC and another one was excluded due to a history of non-hepatic malignancy. Two patients were discovered to have HCC during the first month of treatment. These two patients were excluded as we proposed that HCC actually occurred before DAA initiation. Ten patients were lost to follow-up and 2 patients died during the study period. 531 were followed up for 2 year starting from the end of treatment. Two patients with focal hepatic lesions that remained indeterminate until the end of the 2 years were excluded. The remaining 529 were included in our study. None of them had chronic hepatitis B virus (HBV) infection, human immunodeficiency virus (HIV) infection, or other chronic liver disease and none had a history of alcohol intake.
The study was approved by the ethics committee of Alexandria Faculty of Medicine (IRB no. 00012098). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). Informed consent was obtained from all included subjects. Confidentiality of data was respected and no personal data were identified.
Methods
Patients were assessed prospectively before DAA initiation as regards history, clinical examination, laboratory investigations including complete blood count (CBC), alanine transaminase (ALT), aspartate transaminase (AST), prothrombin time (PT), international normalized ratio (INR), serum albumin, total and direct bilirubin, urea, creatinine, α-fetoprotein (AFP), hepatitis B surface antigen (HBsAg), hepatitis B core antibodies (HBcAb), and quantitative real-time PCR for HCV RNA. Quantitative real-time PCR for HBV DNA was performed if HBsAg and/or HBcAb were positive. Ultrasound abdomen was performed to exclude presence of any focal hepatic lesions and triphasic CT liver scan was performed to characterize focal hepatic lesions if present according to the LI-RADS classification [13]. ARFI was performed for those without clinically evident liver cirrhosis and FIB-4 ≥ 1.45.
DAA regimens were chosen according to the Egyptian national treatment program of hepatitis C guidelines [10]. Quantitative real-time PCR for HCV RNA was performed again 12 weeks after the end of treatment to assess virological response.
Follow-up ultrasound and AFP were performed every 4 months for HCC surveillance. Triphasic CT liver scan was performed for those with any detectable focal hepatic lesion on ultrasound and for patients with AFP ≥ 20 ng/ml [14].
Genotyping for TLL1 rs17047200 was assessed in all patients who developed HCC within 2 years from the end of DAA treatment and in a propensity score matched control group (4 control: 1 case). TaqMan single nucleotide polymorphism genotyping allelic discrimination assay was used. Genotypes were determined by the SDS software. Genomic DNA was extracted using the QIAamp Genomic blood DNA purification kit (QIAGEN, Germany). The TLL1 gene SNP (rs17047200) was detected by SNP genotyping assay (Applied biosystems-Life Technologies, USA) using the Stratagene Rotor-Gene Q real-time PCR system (MX3000P, Qiagen, Germany).
Statistical analysis
IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) was used. The Kolmogorov-Smirnov test was used to verify the normality of distribution. Significance of the obtained results was judged at the 5% level. The chi-square (χ2) test was used for categorical variables. Fisher’s exact or Monte Carlo correction was used for χ2 correction when more than 20% of the cells had an expected count less than 5. Student’s t-test was used for normally distributed quantitative variables. The Mann-Whitney test was used for abnormally distributed quantitative variables to compare between two studied groups.
Results
529 cirrhotic subjects were included in the study, 7 (1.32%) of them developed HCC within 1 year of follow-up and a further 10 patients (1.89%) developed HCC during the second year of follow-up. All of them achieved an SVR. Table 1 shows the characteristics of the studied population. Table 2 shows differences between those who developed HCC and a matched group of control subjects who did not develop HCC regarding data before starting DAAs. The control group was selected based on propensity score matching in the ratio 1 : 4. There were no statistically significant differences between the two groups regarding pretreatment ALT, AST, albumin, AFP and creatinine.
Table 1
Characteristics of the studied population (529 subjects)
Table 2
Comparison between those who developed hepatocellular carcinoma (HCC) and the matched control group who did not develop HCC after DAAs during the 2-year follow-up according to pretreatment parameters
Pretreatment hemoglobin and platelet levels were significantly lower among those who developed HCC later. INR and total bilirubin before starting DAAs were significantly higher among those who developed HCC during the follow-up.
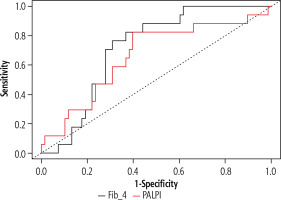
Pretreatment FIB-4 was significantly higher among those who developed HCC than those who did not develop HCC during the 2-year follow-up period. The median FIB-4 in those who developed HCC was 3.31 (IQR = 2.91-4.6) while it was 2.45 (IQR = 1.68-3.35) in the other group (p = 0.038) (Fig. 1 and Table 2).
Regarding the performance of pretreatment FIB-4 for predicting HCC occurrence after DAAs, ROC curve analysis revealed that at a cut-off ≥ 3.07, pretreatment FIB-4 had a sensitivity of 76.5% (95% confidence interval [CI]: 50-93%) and negative predictive value (NPV) of 92% (95% CI: 82-97; area under the curve [AUC] = 0.72, 95% CI: 0.6-0.83, p = 0.006) (Fig. 1).
Pretreatment ALBI score was not significantly different between the groups while pretreatment PALBI score was significantly higher in those who developed HCC. It was –2.48 (–2.53 to –2.26) vs. –2.57 (–2.74 to –2.45) (p = 0.039). Regarding the performance of pretreatment PALBI score for predicting HCC occurrence after DAAs, ROC curve analysis revealed that at a cut-off ≥ –2.5, pretreatment PALBI score had a sensitivity of 82.4% (95% CI: 56.6-96.2), and NPV of 93.2% (95% CI: 82.4-97.4; AUC = 0.66, 95% CI: 0.55-0.76, p = 0.03) (Fig. 1).
Regarding genotyping for TLL1 rs17047200, 38 (55.8%) patients of the control group and 9 (52.9%) patients in the HCC group had AA genotype. 26 (38.2%) patients in the control group and 5 (29.4%) patients in the HCC group had AT genotype, while 4 patients in the control group (5.8%) and another three (17.6%) in the HCC group had TT genotype. These differences were statistically insignificant (χ2 = 2.59, p = 0.27) (Table 3).
Discussion
Before the era of DAAs the annual risk of HCC occurrence in patients with HCV-related cirrhosis was 2-8% [6]. Many studies have suggested that DAA exposure increases the risk of HCC occurrence while other studies suggested that achieving SVR decreases that risk [7].
In our study, only 1.3% of cirrhotic patients who received DAAs developed HCC within 1 year from the end of DAA therapy and 1.9% during the second year.
It is important to identify the predictors of HCC occurrence among cirrhotic patients after initiating HCV treatment using DAAs. There were no statistically significant differences between groups regarding pretreatment ALT, AST, albumin, AFP and creatinine. Pretreatment hemoglobin and platelet levels were significantly lower among those who developed HCC later. INR and total bilirubin before starting DAAs were significantly higher among those who developed HCC during the follow-up.
Interestingly, pretreatment FIB-4 scores were notably higher in those who developed HCC on follow-up. Ioannou et al. [15] and Na et al. [16] reported that annual risk of HCC occurrence after DAAs was higher among those with pretreatment FIB-4 ≥ 3.25. In our study, ROC curve analysis revealed that at a cut-off > 3.07 pretreatment FIB-4 had a sensitivity of 76.5% in predicting HCC occurrence within 2 years from the end of DAA treatment with NPV = 92%. Moreover, pretreatment PALBI score ≥ –2.5 had a sensitivity of 82.4% and NPV of 93.2%.
A genome-wide association study conducted on Japanese patients who had achieved SVR after interferon therapy for HCV infection revealed a strong association between single nucleotide polymorphism (SNP) rs17047200 located within the TLL1 gene and occurrence of HCC [8]. The authors proposed that this SNP can affect splicing of TLL1 mRNA, resulting in short variants with high catalytic activity that accelerates hepatic fibrosis and carcinogenesis.
Another study on Japanese patients who achieved an SVR after DAAs and followed up for at least 1 year from the end of treatment revealed a higher proportion of rs17047200 AT/TT among those who developed HCC. Of note, patients who developed HCC within 1 year from the end of treatment (n = 23) were excluded from this study [17].
On the other hand, an Italian study performed on Caucasian cirrhotic patients who received DAAs from a single center revealed no association between TLL1 genotypes and the incidence of HCC development [18]. Similarly, in our Egyptian cohort there was no statistically significant difference between HCC and non-HCC groups regarding genotyping for TLL1 rs17047200. Similar results on Egyptian patients who developed de novo HCC within 6 months from the end of DAAs were also reported [19].
Strengths of our study include being performed on Egyptian patients where genotype 4 is the predominant HCV genotype [20, 21]. Also they include assessing the PALBI score as a possible predictor for HCC occurrence after DAAs. To our knowledge this is the first study to assess PALBI score for this purpose. Limitations of our study include the small number of patients who developed HCC during the 2-year follow-up period. Long-term follow-up studies to evaluate pretreatment total bilirubin, hemoglobin, FIB-4 and PALBI score as predictors of HCC occurrence after DAAs are recommended.