Introduction
Chronic urticaria (CU) is a debilitating skin condition characterised by recurrent occurrence of hives and/or angioedema for more than 6 weeks. Chronic spontaneous urticaria (CSU) is a subset of CU with unknown triggers, with an incidence rate of ~1.4% per year, and a prevalence of 0.5–5% in the general population [1, 2]. Urticaria inducible through provocative triggers is termed chronic inducible urticaria (CIndU) [2–5]. CU significantly impacts patients’ quality of life (QoL), including a loss in work productivity and interference with daily activities, sleep, and personal relationships, causing high levels of anxiety and psychological distress [6–8]. More than 60% of all patients remain symptomatic on standard doses of second-generation antihistamines (sgAH). Among them, 38-54% do not respond to even 4-fold approved doses of sgAH [9, 10]. For these patients, add-on omalizumab to sgAH is recommended as third-line therapy; followed by add-on cyclosporine for further treatment escalation [4].
Data on demographics, epidemiology and burden of CU, and adherence to modern treatment guidelines for the management of CU in Russian clinical practice settings are scarce. Knowledge of disease burden and treatment patterns can help understand and mitigate undesired outcomes associated with sub-optimal approaches to treat CU. AWARE (A World-wide Antihistamine-Refractory chronic urticaria patient Evaluation) was a real-world study that investigated treatment practices and use of clinical resources by patients refractory to treatment with H1-antihistamines (H1-AH). The first published results of the AWARE study revealed a significant discrepancy between the clinical recommendations for the treatment of patients with CSU and real-world clinical practice in Germany [11]. Subsequent publications from the AWARE study have highlighted the high healthcare utilisation and QoL impairments in patients with CU [12, 13].
Aim
The 2-year data from Russian patients with CU enrolled in the AWARE study are presented here, focusing on their clinical characteristics, treatment patterns, disease activity, utilisation of healthcare resources, and impact on QoL.
Material and methods
Study design
The detailed study design of the AWARE study has been previously published [11]. In short, AWARE was a 2-year, multicentre, non-interventional, prospective observational study of patients with a medically confirmed diagnosis of CU and symptoms uncontrolled by H1-AH. Effects of therapeutic decisions and treatment paradigms were recorded in terms of symptom control, QoL, and healthcare utilisation for a period of 2 years. From Europe, 418 sites from 12 countries (Germany, Spain, United Kingdom, Italy, Greece, Russia, France, Denmark, Belgium, Portugal, Norway, and Sweden) participated in the study. The present report focusses on data from patients enrolled at 6 study sites in different cities in Russia. The study protocol was reviewed and approved by the Independent Interdisciplinary Committee for the Ethical Review of Clinical Studies (http://ethicuni.ru/main.php). The study was conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from each participant.
Study population
Patients aged ≥ 18 years, with a medically confirmed diagnosis of CU for more than 2 months, refractory to H1-AH therapy, and able to give written informed consent were eligible. Patients with anticipated difficulties in follow-ups for the 2-year study period or simultaneous participation in other studies were excluded.
Study outcomes
The co-primary endpoints of the study were the courses of mean patient-reported outcomes (PROs) measuring disease activity using the weekly urticaria activity score (UAS7) and QoL using the dermatology life quality index (DLQI). Secondary endpoints included the number of patients with CSU, CIndU, or both diagnoses (CSU + CIndU); pattern of previous and current medication; frequency of patients in treatment groups by visit; frequency of patients with respect to DLQI categories; extent of healthcare utilisation by disease category; frequency of patients with angioedema and wheals; and patient satisfaction with current therapy on a visual analogue scale (VAS: 0 = not at all satisfied to 10 = very satisfied).
Assessments
Post-baseline visit, 8 quarterly interval follow-up visits were planned. Current treatment/therapy plan, occurrence of angioedema since last visit/the last 6 months, medical resource utilisation, UAS7, DLQI, and existence of inducible urticaria were recorded at every visit. The various observed treatment groups are presented in Supplementary Figure S1. Urticaria activity for 7 consecutive days (UAS7) is scored based on 2 questions answered on Day: 1. How many wheals have appeared? (scored as 0 = 0 wheals, 1 ≤ 20 wheals/24 h, 2 = 20–50 wheals/24 h, or 3 ≥ 50 wheals/24 h); 2. How severe was the itching? (scored as 0 = none, 1 = mild, 2 = moderate, or 3 = intense). Effect on QoL was assessed via the DLQI score categories (0–1 = no effect at all, 2–5 = little effect, 6–10 = moderate effect, 11–20 = large effect, and 21–30 = extremely large effect). Co-morbidities and patient satisfaction were recorded at yearly intervals.
Analysed data were stratified by diagnosed disease subtype: CSU for patients with CSU without angioedema, CSU with angioedema, or angioedema without wheals; CIndU for patients diagnosed with urticaria factitia, cold urticaria, cholinergic urticaria, delayed pressure urticaria, heat urticaria, light/solar urticaria, vibration-induced angioedema, aquagenic urticaria, or contact urticaria; and CSU + CIndU for patients with diagnoses outside of both of the aforementioned groups.
Statistical analysis
Only descriptive analyses were performed. Quantitative data were analysed by the statistical parameters of mean, and standard deviation (SD). Qualitative data were analysed as absolute and relative frequency distributions. The calculation of percentages was based on the valid data per parameter, excluding patients with missing values. Conditional endpoints analysis was based on the number of subjects fulfilling the respective condition. All PRO summary scores and respective sub-scores were processed as quantitative data. In addition, the DLQI and UAS7 were analysed categorically. UAS7 was considered not evaluable if entries for 7 consecutive days were missing. If ≥ 2 questions were unanswered for DLQI measures, the questionnaire was not scored.
Results
Patient disposition
In total, data of 135 patients was collected from 6 study centres (4 allergological and 2 dermatological) in Russia (Moscow, Saint-Petersburg, Smolensk, Rostov on Don, Stavropol and Kazan). One patient was excluded from the analysis due to violation of the inclusion/exclusion criteria. Among the 134 patients included in the analysis, 13 discontinued and 121 completed all study visits. Reasons for discontinuation included loss to follow-up (n = 3), withdrawal of informed consent (n = 5), spontaneous remission of CU (n = 3), and relocation (n = 2).
Patient historical and baseline characteristics
Patients were mostly women with a mean age of 43.7 years for all patients and a duration of urticaria averaging 3.7 years. The majority of patients were diagnosed with CSU (n = 124, 92.5%). Only 8 (6%) patients were diagnosed with CIndU and 2 (1.5%) patients with CSU + CIndU (Table 1). Nearly a quarter (23.1%) of all CU patients were obese (body mass index (BMI) > 30 kg/m²), 75.4% of patients had either a normal BMI or were moderately overweight (BMI > 18.5 kg/m² to ≤ 30 kg/m²), and 1.5% of patients were underweight (BMI ≤ 18.5 kg/m²; Table 1). At baseline, hypertension (23.9%), allergic rhinitis (17.9%), and obesity (17.2%) were the most frequently reported co-morbidities (Table 1).
Table 1
Baseline demographics and disease characteristics of patients with CU
[i] BMI – body mass index, CIndU – chronic inducible urticaria, CSU – chronic spontaneous urticaria, CU – chronic urticaria, DLQI – dermatology life quality index, ER – emergency room, HCU – healthcare utilisation, N – total number of patients, n – number of patients, SD – standard deviation, UAS7 – weekly urticaria activity score.
Treatment patterns were non-adherent to guidelines prior to the study
Any prior medication for urticaria was recorded in 71.6% of CU patients, with non-sedative antihistamines (nsAH) the most frequently prescribed in 61.9% of patients, followed by sedative antihistamines (sAH) and glucocorticosteroids, both prescribed in 33.6% of patients. Prior to the baseline visit, 28.4% of all patients were untreated for urticaria (Table 2), and only 35.1% of patients were on 2014 EAACI/GA2LEN/EDF/WAO urticaria guideline-recommended treatments (16.4% on first-line approved nsAH; 7.5% on second-line up dosed nsAH; 6.7% and 4.5% on third-line leukotriene inhibitor montelukast and anti-IgE antibody, omalizumab, respectively). No patient was prescribed the alternative third-line treatment, cyclosporine A.
Table 2
Prior medication for urticaria at baseline by diagnostic groups
| Treatment | CSU (n = 124) | CIndU (n = 8) | CSU + CIndU (n = 2) | Total (n = 134) |
|---|---|---|---|---|
| Any treatment: | 89 (71.8) | 5 (62.5) | 2 (100.0) | 96 (71.6) |
| nsAH | 78 (62.9) | 4 (50.0) | 1 (50.0) | 83 (61.9) |
| sAH | 45 (36.3) | – | – | 45 (33.6) |
| Corticosteroid | 44 (35.5) | 1 (12.5) | – | 45 (33.6) |
| Other | 16 (12.9) | – | – | 16 (11.9) |
| Montelukast | 11 (8.9) | – | 1 (50.0) | 12 (9.0) |
| Omalizumab | 7 (5.6) | – | – | 7 (5.2) |
| Ketotifen | 5 (4.0) | – | 1 (50.0) | 6 (4.5) |
| Plasmapheresis | 2 (1.6) | – | 1 (50.0) | 3 (2.2) |
| Autologous whole blood injection | 1 (0.8) | 1 (12.5) | – | 2 (1.5) |
| Ranitidine | 1 (0.8) | – | 1 (50.0) | 2 (1.5) |
| Cyclosporine | 1 (0.8) | – | – | 1 (0.7) |
| Hydroxychloroquine | 1 (0.8) | – | – | 1 (0.7) |
| Treatments for urticaria†: | ||||
| No treatment | 35 (28.2) | 3 (37.5) | – | 38 (28.4) |
| Combination of nsAH and sAH | 37 (29.8) | – | – | 37 (27.6) |
| Approved nsAH | 20 (16.1) | 2 (25.0) | – | 22 (16.4) |
| Up-dosed nsAH | 8 (6.5) | 1 (12.5) | 1 (50.0) | 10 (7.5) |
| Montelukast | 8 (6.5) | – | 1 (50.0) | 9 (6.7) |
| Omalizumab | 6 (4.8) | – | – | 6 (4.5) |
| Other | 5 (4.0) | 1 (12.5) | – | 6 (4.5) |
| sAH | 4 (3.2) | – | – | 4 (3.0) |
| On-demand nsAH | 1 (0.8) | 1 (12.5) | – | 2 (1.5) |
| Cyclosporine | – | – | – | – |
More than a quarter of CSU patients (28.2%) did not receive any treatment for urticaria prior to baseline, and treatments not recommended by guidelines were prescribed in more than a third (37%) of them. A combination of sAH and nsAH was the most frequently prescribed non-recommended treatment, followed by other non-recommended treatments and sAH (Table 2) [14]. A decrease in the use of sAH was marked during the observation period, with only 0.9% of patients treated with sAH at the end of the study. However, there was an increase in the use of other non-recommended therapies from 4% prior to baseline to 11.0% at the end of the study (Figure 1).
Figure 1
Average weekly urticaria activity score (UAS7) and dermatology life quality index (DLQI) total scores, and the corresponding change in the treatment for urticaria over the 2-year observation period in patients diagnosed with CSU. Other therapies include other treatment combinations, ketotifen, plasmapheresis, autologous whole blood injection, ranitidine, and hydroxychloroquine
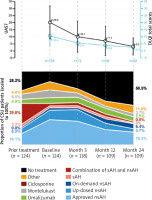
In total, 97.0% of CU patients were treated with medication during the study period. The majority of all patients (89.6%) were treated with nsAH, and 25.4% with montelukast. Omalizumab was administered at least once in 14.2% of patients during the study. At the end of the 2-year observation period, more than half of the CSU patients were not treated for urticaria and the proportion of patients on non-recommended treatments decreased to 12% (Figure 1).
Disease activity and QoL showed improvements with a shifting treatment approach
For patients with CSU who used the UAS7 diary, moderate disease activity at baseline, indicated by a mean ± SD UAS7 of 20.2 ±11.7 (n = 124), improved to mild severity by the end of 2 years with a mean ± SD score of 3.2 ±6.1 (n = 109) (Figure 1).
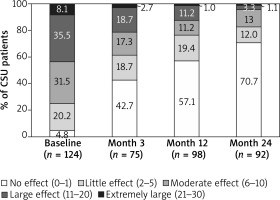
The mean ± SD DLQI score of 10.2 ±6.5 for all patients showed a moderate effect of disease on QoL at baseline, which improved by the end of the study with a score of 2.2 ±4.1. Similarly, for CSU patients, the mean ± SD DLQI score improved from 10.3 ±6.4 at baseline to 2.3 ±4.2 by the end of the study (Figure 1). Categorical DLQI scores at baseline for patients with CSU showed that 43.5% of patients had a large to extremely large impact on their QoL related to urticaria (DLQI score ≥ 11; Figure 2). After 1 year of observation, improvement in QoL was observed, and only 12.2% of CSU patients reported a large to extremely large impact on their QoL. At 2 years, the proportion of patients with a large to extremely large impact on their QoL further decreased to 4.3%. Conversely, patients reporting no effect of urticaria on their QoL increased from 4.8% at baseline to 70.7% after the 2-year observation period (Figure 2).
Angioedema and wheals improved over the course of the study
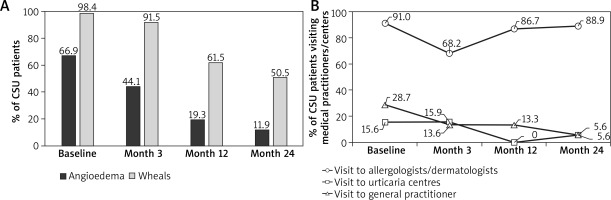
Overall, 85 (63.4%) patients with CSU had angioedema. For 6 months prior to baseline, 66.9% of CSU patients and 67.2% of all patients reported angioedema (Table 1). Moderate-to-severe angioedema was reported by 46.7% of patients. Angioedema was experienced for a mean ± SD duration of 40.2 ±91.9 h by all patients. At baseline, 98.5% of patients in all diagnostic groups reported wheals, currently or during the last 6 months. For patients diagnosed with CSU, 98.4% reported wheals at baseline. Angioedema and wheals considerably improved over the observation period to 11.9% and 50.5% of CSU patients, respectively, at the end of the study (Figure 3 A).
Patients’ satisfaction with therapy improved during the study
Patient satisfaction recorded by VAS showed a modest satisfaction with the current therapy at baseline, with a score of 4.4 (n = 89). The patient satisfaction VAS score increased to 7.0 (n = 84) at Year 1, but showed a small dip at Year 2 with a score of 6.6 (n = 63).
Healthcare resource utilisation was high in patients with CSU
Prior to the baseline visit, 17.4% of all patients visited an emergency physician or emergency room (ER), 53.0% of patients were hospitalised at least once, 27.3% visited a general practitioner, 14.4% visited a specialised urticaria centre, and 91.7% visited an allergologist or dermatologist (Table 1). At baseline, 28.4% of all patients reported at least one sick leave due to urticaria since the time of diagnosis, with a mean duration of 18.4 weeks of leave. During the study period, only 5 instances of sick leave were reported by all CU patients (2 patients at the Month 3 follow-up, 2 patients at Year 1, and 1 patient at Year 2), and no ER visit was reported. Three hospitalisations at Month 3, and one hospitalisation each at Year 1 and Year 2 was reported. Visits to specialised urticaria centres and general practitioners, but not to allergologists or dermatologists, decreased during the study period (Figure 3 B).
Discussion
A large majority of patients (92.5%) in the Russian cohort of the AWARE study were diagnosed with CSU. A low rate of CIndU (6%), and even lower rate of CSU + CIndU (1.5%), was diagnosed in this cohort. This observation is in contrast to previous publications that reported as high as 24% of patients diagnosed with CSU + CIndU from other regions in Europe [4, 11, 13]. Since allergologists or dermatologists in specialised medical centres conducted the diagnosis in this cohort, it is likely that the differential diagnosis of CU was done with high accuracy. A high proportion of patients with co-morbid obesity in the present study may also contribute to the higher diagnosis of CSU [15].
Data for the Russian cohort of the AWARE study were collected between January 2015 and July 2017. During this period, the treatment guidelines for urticaria were defined by the 2014 EAACI/GA2LEN/EDF/WAO urticaria guideline [14], which recommended sgAH as first-line therapy, followed by escalation up to 4 times the approved dose as second-line therapy. Cyclosporine A, montelukast or omalizumab were recommended as the third-line add-on therapy. The guidelines discouraged the long-term use of glucocorticosteroids or first-generation H1AH/sAH. However, the revised EAACI/GA2LEN/EDF/WAO urticaria guideline in 2018, recommended omalizumab as the standard third-line therapy before cyclosporine. A short course of glucocorticosteroids is permitted in cases of severe exacerbation of CSU [14].
Similar to previous reports from the AWARE study, a large percentage of patients were undertreated for urticaria and many were not treated, in accordance with the guidelines [11, 13]. More than a quarter of patients with CSU received no treatment for urticaria, and more than a third received non-recommended therapies. In total, 33% of patients used sAH, which was strikingly higher than the 10% and 3.2% reported in the German and the Scandinavian cohorts of the AWARE study, respectively [11, 13]. A possible reason for the high use of sAH, in combination with approved nsAH, could be its use as emergency medication for angioedema or as a sedative anxiolytic. The number of patients whose treatment was escalated from the approved dose to increased dose of nsAH was low across all visits. Physician and patient preferences may play a role in the infrequent up-dosing of nsAH. While physicians may be apprehensive of prescribing off-label dosages or of conflicting and limited evidence for efficacy of up-dosing nsAH, patients have concerns about side effects, loss of efficacy, or dependency associated with up-dosing nsH1AH [9, 16]. Adherence to treatment may also play an important role in assessing therapeutic approaches. The strongest changes in medication intake during the study period were observed for up-dosed nsAH (11.9% at baseline, 3.4% at Month 24) and nsAH (35.8% at baseline, 18.1% at Month 24) with an increase in the number of patients taking nsAH on demand from baseline.
The use of glucocorticosteroids, reported in 35.5% of patients with CSU, was higher than the German (15.8%) and Scandinavian (19.0%) cohorts of the AWARE study [11, 13]. This may reflect a higher exacerbation rate of undertreated CU in Russia. Omalizumab is the only biologic approved for use in patients with CSU who remain symptomatic despite H1-AH treatment and it is currently the only licensed third-line treatment [14, 17]. Several randomised controlled trials and real-world evidence data have shown that omalizumab effectively controls the symptoms of CSU, reduces the frequency and severity of angioedema in H1-AH-refractory CSU, and improves QoL [18–22]. The low proportion of patients escalating to omalizumab treatment in Russia can be explained by the inaccessibility and high cost of omalizumab until 2017 to the majority of patients, and the absence of reimbursement programmes during the AWARE study. Escalation of therapy in favour of montelukast and systemic glucocorticosteroids, in accordance with the consensus at that time, was due to the affordable cost. Patients with CSU in the study were not prescribed cyclosporine or other immunosuppressants due to safety concerns and inefficiency in angioedema [23] that were present in more than 67% of patients. A radical decrease in the use of sAH, and a shift in the treatment paradigm towards management and treatment escalation more in line with the treatment guidelines were observed from baseline. This may have been responsible for the improvement in disease activity burden, and QoL observed during the course of the study. Since CSU is a self-limiting disease, some patients may have experienced spontaneous remission of symptoms and some may have benefitted from proper treatment escalation during the study. This likely contributed to more than half of the patients not being treated at the end of the study.
Due to low rates of treatment and non-recommended treatment escalations prior to baseline, the burden of angioedema and wheals was high with moderate-to-severe disease activity. Concomitant angioedema and wheals decreased over the 2 years of the observation period. The plunge in these symptoms corresponds well with improvements in disease activity score and QoL assessed throughout the observation period. However, a considerable number of patients still had high disease burden after 2 years.
Compared with the cohorts of other European countries, the Russian cohort of the AWARE study used only 3 PROs: UAS7, DLQI, and VAS to demonstrate the severity, clinical course, and QoL controllability of CU. The baseline disease activity and burden on QoL reported in the current study was higher than that reported in the German or Scandinavian cohort of the AWARE study [11, 13]. However, within the Scandinavian cohort, the impact of urticaria on QoL was higher in patients from Norway than that reported here. At least a moderate impact of urticaria on QoL was observed in more than 75% of patients, with 43.6% of patients reporting a large to extremely large impact on their QoL. This burden of disease was higher than the 32.8% of patients in the German cohort reporting a large to extremely large impact of urticaria on QoL [11]. Even though patients showed modest satisfaction with the current therapy at baseline, only 6.8% of patients stated that CU had no impact on their QoL.
Utilisation of healthcare resources for CU was high in Russia. An overwhelming majority of patients consulted specialist allergologists or dermatologists. Over the course of the observation period, the frequency of consultations with specialists did not change considerably, while the number of patients visiting urticaria centres and general practitioners decreased over time. The rate of hospitalisations was also very high at baseline with more than half of the study population experiencing at least one prior hospitalisation. This may be related to the undertreatment-linked exacerbations in many cases at baseline.
At baseline, more than a quarter of all patients from Russia with CU had, on average, reported more than 18 weeks of sick leave due to urticaria since the time of diagnosis, indicating a considerable socioeconomic impact. In comparison, 27% of patients in Germany reported sick leaves due to urticaria with an average duration of 9 weeks and 25.9% of patients in Scandinavia reported sick leaves due to urticaria with an average duration of 3.8 weeks/year. Previous publications have also suggested a high frequency of absenteeism in patients with CU [6, 24]. However, during the study patients rarely took sick leave in Russia, apparently due to decrease in the emergence of angioedema.
Conclusions
The present data show that untreated or sub-optimally treated patients with CU have a high disease burden, contributing to increased economic burden and utilisation of healthcare resources. Following the guideline-based approach to treatment and managing patients with appropriate treatment escalation can improve patient care and disease outcomes.