Introduction
Haemangiomas are benign tumours resulting from abnormal proliferation of endothelial cells, which occurs particularly often in early childhood. Treatment is required because they can lead to ulcers or distortions within the area of their occurrence. A highly effective method of treatment is excision surgery of the lesion however conservative treatment, including pharmacotherapy with propranolol, leads to good results as well. This β-blocker, widely used in the treatment of cardiological and psychiatric diseases, causes vasoconstriction and blocks the activity of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF). It also causes induction of apoptosis within the endothelium, which results in a reduction in the size of the haemangioma. In our article, we discuss the results of propranolol treatment in a group of 108 patients [1–3].
Aim
Haemangioma, one of the most common benign neoplasms of early childhood, is a significant clinical problem due to cosmetic reasons but also because of possible health complications. The main objectives of this article are to present a group of patients who received propranolol treatment and then discuss the results of pharmacotherapy, its efficacy and safety, as well as conclusions based on this treatment strategy for haemangiomas.
Material and methods
In 2013–2018, 108 patients with IHs were multidisciplinary diagnosed and treated in the department of paediatric surgery as well as in the department of paediatric cardiology. All patients underwent ECG and echocardiographic examinations in order to exclude underlying cardiologic conditions. No associated heart structural defects nor cardiac rhythm disturbances were found. Seventy-seven (71.3%) were girls and 31 (28.7%) were boys.
At the start of treatment the patients were 2 to 21 months old. Most of them (n = 17, 15.7%) were 5 months old. Thirty-two were older than 6 months (29.6%) while only 6 patients were older than 12 months (5.6%). The average age was 6.87 months.
Haemangiomas were located in different body areas. Multiple lesions, occupying more than one area, occurred very often (44 cases, 40.7%). The head was the most common localization (n = 73, 67.6%) of both single and multiple changes. Haemangiomas on the scalp itself, excluding the face area, occurred in 33 (30.6%) cases. Table 1 presents the location of haemangiomas. Twenty (18.5%) patients had ulcerations or were bleeding from tumours and 6 (5.6%) presented components of arteriovenous fistulas.
Table 1
Location of haemangiomas on patients’ bodies
| Location of the haemangioma | Number of patients |
|---|---|
| Head | 12 |
| Orbit | 10 |
| Face | 6 |
| Lip red | 5 |
| Chest | 5 |
| Upper limb | 5 |
| Nose | 5 |
| Lower limb | 3 |
| Groin | 2 |
| Stomach | 2 |
| Neck | 2 |
| Salivary glands | 2 |
| Shoulder | 1 |
| Scrotum | 1 |
| Buttock | 1 |
| Ear | 1 |
| Nape/back | 1 |
| Multiple changes* | 44 |
Ultrasound imaging was used to evaluate each patient and allowed assessment of vascular flows and the presence of fistulas. Computed tomography (CT) or magnetic resonance imaging (MRI) was also used in individual cases. Coagulation parameters and other laboratory tests were also analysed. The most common observed changes, present in 82 (75.9%) cases, were abnormal values of fibrinogen (56 cases, 51.9%), D-dimer (32 cases, 29.6%), activated partial thromboplastin time (APTT) (30 cases, 27.8%), platelets (PLT) (29 cases, 26.9%) and lactate dehydrogenase (LDH) (9 cases, 8.3%).
All of the patients (n = 108) had propranolol pharmacotherapy. The vast majority of patients were hospitalized for 3 to 4 days, followed by continued treatment with monitoring in the outpatient clinic. The initial dose of propranolol was 0.5 to 1.5 mg/kg/day. On the day of discharge from the hospital, the most common dose was 1.5 to 2 mg/kg/day. The maximum dose – 3 mg/kg/day – was reached after 3 to 5 months of treatment. In 86% of patients, β-blocker therapy lasted longer than 12 months. The average time for study participants who finished or discontinued the treatment was 12.42 months.
The first follow-up visit in the Cardiology Clinic took place a month after the treatment. Further follow-up outpatients visits were conducted in accordance with a schedule set by the cardiologist. ECGs were routinely performed during these visits.
In the Paediatric Surgery Outpatient Clinic, follow-up visits were scheduled every 2–3 months, until the end of pharmacotherapy.
Results
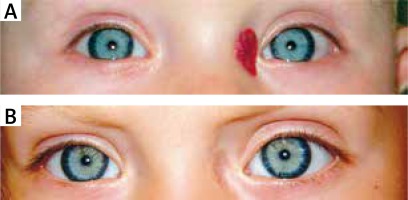
Pharmacotherapy with propranolol appeared to be effective (Figures 1 A, B). Regression of changes (rated based on subjective assessment of parents and doctors) was observed in 103 (95.37%) patients. In 5 (4.6%) patients, despite the implementation of treatment, there was progression of lesions (to remove haemangiomas, 3 (2.8%) patients underwent surgery and 2 (1.9%) laser therapy). Propranolol therapy resulted in reduction of haemangioma size in 11 (10.2%) patients who, nevertheless, had surgical removal of the lesion on their parents’ request. Changes in haemangioma dimensions were assessed by ultrasound examination and compared to patients’ medical history. After treatment with propranolol, only 16 (14.8%) patients showed abnormalities of coagulation parameters and laboratory tests (compared to 82 patients before the treatment): D-dimers (6 cases, 5.6%), fibrinogen (3 cases, 2.8%), APTT (1 case, 0.9%), LDH (5 cases, 4.6%) and PLT (3 cases, 2.8%).
Figure 1
Effect of propranolol therapy: A – before treatment, B – after propranolol treatment. Note cosmetically and functionally important region (orbit)
Side effects of the treatment were observed in 21 (19.44%) patients. The most common side effects were night anxiety and nightmares (n = 12, 11.1%), somnolence (n = 2, 1.9%), excessive sweating (n = 2, 1.9%), short episode of respiratory arrest (n = 2, 1.9%), hypoglycaemia (n = 2, 1.9%), and loose stools (n = 1, 0.9%). A full list of side effects is presented in Table 2.
Discussion
Infantile haemangioma is the most common benign neoplastic lesion of newborns, occurring in 2.6% to 4.5% of children younger than 1 year. Most of them are single focal lesions. Predisposing factors include premature delivery, low birth weight (less than 1500 g), female sex, and Caucasian race. In most patients, this lesion disappears spontaneously although in 5% to 10% of cases special treatment is necessary. The need for special treatment is appropriate when the possibility of serious complications exists, including excessive growth or threatening location of the lesion which can lead to visual impairment (amblyopia, astigmatism), ulceration, irreversible deformity, or airway obstruction. In such cases, β-blockers, in particular propranolol, are the treatment of choice [4–6].
In March 2014, a meeting of European experts on infantile haemangioma took place in Castres, France. This panel of experts established treatment standards for this condition and published them a year later in the European Journal of Pediatrics. Hoeger et al. had determined that oral propranolol therapy is the best therapeutic option for treatment of these lesions and thus appropriate first-line therapy [5]. Treatment with this β-blocker was first described by Léauté-Labrèze et al. in 2008 [7]. Outpatient treatment is allowed; in such a case the starting dose is 1 mg/kg/day, with increases every week until the maximum dose of 2 to 3 mg/kg/day is achieved. In the case of inpatient treatment, therapy should be started at 1 mg/kg/day and then cardiologic assessment should be performed at 1 and 2 h following administration. If the patient tolerates medication, the dose can be increased to 2 mg/kg/day on the second day of therapy. In most published studies, the standard therapeutic dose of propranolol was 2 to 3 mg/kg/day. Léauté-Labrèze et al. published in 2015 the results of their study on a group of 460 patients, 456 of whom received treatment. They confirmed the effectiveness of therapy with propranolol at a dose of 3 mg/kg/day for 6 months. Due to the lack of accurate studies comparing the 2 mg/kg/day dose with the 3 mg/kg/day dose, the recommended dose by the European expert group is 2 to 3 mg/kg/day. The drug should be administered in two batches, in doses of 0.5 to 3 mg/kg and the preferred interval between doses should be a minimum of 9 h. The standard length of therapy with propranolol is estimated at 6 months; however, in some cases, the treatment may be extended. Due to the child’s weight increase during the treatment, it is recommended to adjust the total daily dose of the drug every 4 weeks [5, 8].
In 2015, the same European expert group also published guidelines about other available options of treatment of haemangioma. These guidelines include local topical therapy with β-blockers, surgical intervention, and systemic treatment with corticosteroids. Topical use of β-blockers (propranolol in particular) is an alternative method of treatment, however due to the risk of percutaneous resorption of the drug and thus the lack of first-pass effect (the substance bypasses the portal circulation and detoxification by the liver), which ultimately leads to hypotension and bradycardia, it cannot be considered as first-line treatment. Surgical treatment was considered a necessity for many years but retrospective studies have proved that both laser surgery and cryosurgery are less effective methods than using propranolol. Surgery, however, is an important element of reconstruction of the area after lesion removal, primarily by removing excess skin after drug treatment and residual changes. Retrospective studies have shown that systemic treatment with corticosteroids, comparing to propranolol, showed less effectiveness and slower effect of the steroid drugs; they were also poorly tolerated by patients receiving therapy [5, 9, 10].
In our study a small number of patients underwent surgical removal of the lesions. The eligibility criteria for surgery were the observation of IHs progression despite propranolol therapy (3 patients – 2.8%) and clear parents’ request to prevent their children stigmatization in the preschool age (11 patients – 10.2%).
Propranolol, which belongs to a group of non-selective β-blockers, is used in the treatment of infantile haemangiomas but the drug’s exact mechanism of action on these lesions is unknown. This drug is administered orally, which is why it undergoes the first-pass effect, meaning only about 25% of the orally administered drug eventually gets into the bloodstream. The therapeutic effect of this substance relies on vasoconstriction, which is manifested by immediate softening and a change in the colour of the lesion [4, 7, 11]. There are three phases of haemangioma development: proliferation phase, involution phase, and regression phase [4]. The first stage involves basic fibroblast growth factor (bFGF/FGF2/FGF-β) and VEGF, which are the main factors stimulating angiogenesis; the excessive expression of these factors is found in infantile haemangiomas. Propranolol, by reducing the activity (down-regulation) of the rapidly accelerated fibrosarcoma (RAF) protein pathway, and thus the MAPK (mitogen-activated protein kinase) pathway, leads to a decrease in the expression of genes for bFGF and VEGF, which ultimately results in the death of vascular endothelial cells due to apoptosis [4, 7]. It is suggested that the use of this drug leads to other slower, long-term effects. Propranolol may affect pericytes (undifferentiated stem cells), which are involved in regulation of the tone of small blood vessels. This drug, as an antagonist for β2-receptors, blocks catecholamine-induced relaxation of pericytes [4]. This non-selective β-blocker is a lipophilic substance, which allows it to cross the blood-brain barrier, thereby affecting the central nervous system. Use of this drug, and its side effects, can lead to mood changes and may have a negative impact on sleep quality [4].
Other β-blockers used to treat infantile haemangiomas are acebutolol, nadolol and atenolol. These drugs are not lipophilic thus they do not penetrate the blood-brain barrier and, as a result, their use does not lead to side effects directly related to the central nervous system. Compared to propranolol, there are not enough scientific publications to clearly assess their safety and efficacy [9]. Ji et al. published in 2015 the results of studies on the effectiveness of atenolol in the treatment of infantile haemangioma. This drug was administered for 24 weeks in a single dose of 1 mg/kg/day. The research team confirmed the safety and effectiveness of this drug and thus proved that it can be used as an alternative therapy [12]. Atenolol is a selective β-blocker for β1-receptors, which means that its use does not lead to blockage of β2-receptors in the lungs therefore, use of this drug, when compared to propranolol (non-selective β-blocker), reduces the risk of bronchospasm. In addition, atenolol is a drug that is administered only once a day, while propranolol is administered in two separated doses, with a minimum interval of 9 h [4, 5, 9].
In our clinical trial, we chose propranolol as the treatment method, which in 2014, at a meeting of the European expert group, was recognized as the first-line drug in the treatment of IH. The maximum dose of 3 mg/kg/day was used in accordance with European guidelines published in 2015. In our study, the average length of treatment was 12.42 months, which is more than twice as long as the standard time of therapy. Despite available recommendations suggesting termination of propranolol therapy after 1 year of age, nonetheless the decision is reserved for the treatment supervising physician and the treatment could be extended up to 2 years of age [13].
Schupp et al. in 2011 published the results of IH treatment with propranolol on a group of 55 patients (mean age at the start of the treatment was 6 months). The dose they used was 2 mg/kg/day which was 1 mg lower than the dose we used. The average length of treatment was 6 months, which is about half the duration of our study. Thirteen (21.7%) patients experienced adverse effects, but these were not life-threatening episodes. A total of 54 (98.2%) patients showed at least partial response to the treatment [14].
In 2011, Bagazgoitia et al. presented results of their study on a group of 71 patients (mean age: 5.8 months) from 4 different hospitals in Spain and Argentina, who were administered propranolol as a treatment of IH. The dose they used was 1 mg/kg/12 h (2 mg/kg/day in total) and was 1 mg lower than the one we used. The treatment lasted on average 20 weeks and the treatment effectiveness was 98.6% (due to inefficiency, 1 patient did not complete the 12-week period of study) [15].
Zvulunov et al. conducted a study on the effectiveness of propranolol in the treatment of IH on a group of 49 patients (of which 42 were later analysed), which they published in 2011. The average age of patients was 28 months (median: 22 months), and the treatment lasted on average 3.6 months, which is almost twice as short as in our study. The therapy was discontinued when the cession of residual lesions occurred. Patients received the drug in a dose of 1.5–3 mg/kg/day (average: 2.1 mg/kg/day). Treatment effectiveness was measured using Visual Analog Scale (VAS) score, which was reduced from the initial 6.8 to 2.6 at the end of therapy [16].
A comparison of our study with other studies mentioned above is presented in Table 3.
Table 3
Comparison of our study with other studies about treatment of infantile haemangioma with propranolol
Similarly to studies of Zvulunov et al. and of Ali et al., in our study a small number of patients enrolled to propranolol treatment was in the age over 12 months of life [16, 17]. Although the study of Ali et al. has shown, that in this group of patients, there was the highest percentage of non-responders as well as there was the lowest percentage of good-responders (80% and 14.3% respectively), the results did not show that older patients do not respond to propranolol at all [17]. Additionally in the PaNaMa recommendations, the patient’s age over 12 months is a relative contraindication to propranolol therapy [13]. In our study, the IHs in the group of enrolled patients aged more than 12 months, have shown morphology suggesting the active phase of haemangioma with high blood fulfilment of the lesion. The above statement was based on clinical and sonographic assessment. In this group of patients we observed partial/good response in each of enrolled patients. One should conclude that there are no available studies analysing IHs morphology (or other feature) which is connected with a substantial effectiveness of propranolol treatment in older children. In our opinion, in selected cases it is worth introducing propranolol therapy as a first-line treatment also in patients aged more than 12 months.
Conclusions
Treatment of haemangiomas with propranolol is a highly effective method. In our group of patients, regression of changes occurred in more than 95% of cases, and in many patients the abnormalities of coagulation parameters in laboratory tests also regressed. In comparison to the PaNaMa criteria, propranolol was used in a maximum possible dose of 3 mg/kg/day (according to the criteria, maximum dose is 2 to 3 mg/kg/day). In addition, the maximum dose was achieved much slower than in the recommendations, which suggests administration of maximum dose while still hospitalized or during the first 3 weeks of outpatient pharmacotherapy. Use of the drug in lower doses and achieving the maximum dose after 3 to 5 weeks of treatment was not associated with the need for longer pharmacotherapy nor with decreased efficacy of treatment [13].
Although some patients presented side effects induced by propranolol, it seems to be a safer solution than previously used conservative treatment of infantile haemangiomas. The most serious side effect, which was a short episode of respiratory arrest, did not cause any further complications or harmful effects on children’s health and occurred in only 2 cases among the whole group of 108 patients.








