Introduction
Nowadays, kidney transplantation is the preferred treatment for end-stage renal disease as it significantly improves quality of life [1] and increases life expectancy [2] compared to dialysis.
Transplant recipients must adapt to numerous side-effects associated with post-transplant immunosuppression. Many of these align with those commonly observed in the context of immunosuppressive therapies, including increased risk of malignancies and infections. The type and frequency of side-effects are dependent on time and the dose of immunosuppressive drugs.
Although cutaneous malignancies associated with solid organ transplantation are well described in the transplant literature, there are limited data regarding trichological problems after transplantation. Drug toxicity can be a source of hair pathology in solid organ transplant recipients (SOTRs).
Side-effects of medications can lead to ignoring the treatment or non-compliance by patients. Alopecia is a condition that is often detected by patients before it is recognized by clinicians [3]. Quality of life, especially in a female group experiencing alopecia can be significantly reduced. Physicians taking care of transplant recipients should recognize symptoms of drug-induced hair toxicity to minimize these effects.
In most studies the particular type of alopecia was not specified and the researchers refrained from providing details on the characteristics of the hair loss. It should be noted that one of the most common causes of hair loss is androgenic alopecia (AGA) which is a genetically determined condition that develops due to an excessive response to androgens [4]. The other type is telogen effluvium which refers to the excessive loss of hair in the resting (telogen) phase due to factors such as metabolic stress, hormonal fluctuations, or medication use [5]. Whereas anagen effluvium is a condition in which affected anagen hairs experience a toxic or inflammatory injury, leading to the fracture of the hair shaft [6]. Another type of nonscarring hair loss is alopecia areata (AA) which can vary from small patches which may be small (< 1 cm) to very large, to complete hair loss on the scalp (alopecia totalis – AT) or the loss of hair on the scalp, face, and body (alopecia universalis – AU) [7].
In this review, the effects of calcineurin inhibitors (tacrolimus (TAC) and cyclosporine (CsA)), mTOR inhibitors (sirolimus (SIR) and everolimus (EVE)) and other drugs (like azathioprine (AZA) or mycophenolate mofetil (MMF)) on hair condition were analysed.
Tacrolimus, for example, has been associated with alopecia, particularly in kidney-pancreas transplant recipients [8], while CsA has been linked to hypertrichosis [9–16]. The mechanisms underlying these effects are not fully elucidated, but there are some hypotheses including vascular disruption and autoimmune reactions.
Aim
This review aims to synthesize available information on hair disorders in transplant recipients undergoing immunosuppression, by consolidating data from various published sources.
Correlation between immunosuppressive medications and trichological disorders
Calcineurin inhibitors: tacrolimus and cyclosporine
The mechanism of alopecia associated with calcineurin inhibitors has not been described yet. However, it has been postulated that AA involves an autoimmune pathology characterized by activated CD4+ T-lymphocytes. These lymphocytes penetrate the hair follicles, as proposed by Hequet et al. in their study [17]. However, CsA inhibits CD4+ T-lymphocytes which are used in the treatment of AA.
Tacrolimus is a macrolide molecule that inhibits expression of interleukin 2 in T lymphocytes.
Frequently reported side-effects associated with this drug include neurotoxicity, nephrotoxicity and glycaemic disturbances.
TAC-related alopecia was described for the first time in the literature by Shapiro et al. in 1990 [18]. Their research reported an incidence rate of alopecia in this patient cohort ranging from 3% to 6%.
In the above-mentioned study the examination of the association between immunosuppressive medications and trichological disorders primarily relied on the analysis of symptoms reported by patients.
The analysis by Peters et al. was also conducted on the basis of symptoms reported by patients. They suggested that alopecia occurred more frequently in the group of patients receiving TAC (56%) in comparison to patients receiving CsA (30%) [19].
Another extensive trial involving renal transplant recipients disclosed that over 10% of patients treated with TAC experienced alopecia. The particular type of alopecia was not specified, and the researchers refrained from providing details on the characteristics of the hair loss. Their documentation only categorizes alopecia as one of the reported adverse effects [20].
A recent study conducted by Meera et al. in India from January to June 2020 focused on a cohort of 77 patients who underwent renal transplantation and received a TAC-based regimen. The research revealed that nearly 25% of the renal transplant recipients experienced alopecia as one of the adverse effects associated with TAC. However, the specific type of hair loss was unidentified [21].
Tricot et al. conducted a study in a group of 58 kidney-pancreas transplant recipients (27 females and 31 males) [8]. The authors evaluated the incidence of alopecia caused by immunosuppressive drugs. Patients were receiving TAC, MMF, CsA or AZA. Clinically significant alopecia occurred in 28.9% of patients receiving TAC compared to none receiving CsA. Interestingly, all patients who experienced alopecia were also on MMF. However, those taking MMF alongside CsA did not develop alopecia. In the group that developed alopecia, 11 were female and 2 were male. Other causes of alopecia such as viral infections, iron anomalies and dysthyroidism were excluded. Researchers claimed a high incidence of alopecia in female kidney-pancreas recipients treated by TAC. It was reversible by conversion of TAC to CsA in almost all cases. The mean delay between transplantation and alopecia was 422 days (range: 100–1567). In this study a diagnosis of alopecia was suspected following spontaneous reporting by the patient. Subsequently, patients were directed to undergo evaluations by a dermatologist who conducted all further assessments. At intervals of 3 months and annually post-transplantation, patients underwent systematic reviews by a dermatologist to assess skin lesions and alopecia. The clinical diagnosis of alopecia was confirmed through the observation of abnormal hair loss.
In another small prospective study involving living donor liver transplantation recipients treated with TAC, alopecia became the most common side-effect, occurring at a rate of 9.7% [22]. The specific type of alopecia remained undisclosed, the researchers refrained from providing details on the characteristics of the hair loss. Their documentation only categorized alopecia as one of the reported adverse effects.
Alatas et al. performed an analysis about alopecia in children following living related liver transplantation [23]. This study followed 111 children after liver transplantation in 1996–2018, receiving TAC therapy. Alopecia occurred in 2.7% of patients. Alopecia cases were recorded and retrospectively analysed. In all cases, there were no risk factors affecting graft function and TAC was used in appropriate doses. Alopecia was treated with topical corticosteroids and topical TAC.
The alternative analysis suggests that if discontinuing TAC is not possible, using topical minoxidil 5% foam at a dose of 1 ml twice daily might be an effective treatment for alopecia induced by TAC [8].
Some researchers reported that alopecia may be reversed by lowering the dose of TAC [18].
The mechanism of alopecia induced by TAC is not clear.
It is possible that vasoconstriction caused by TAC is associated with alopecia. One of the studies showed that alopecia was caused by disruption of microvascular blood flow to hair follicles. One of the side- effects of TAC was disruption of the vascular endothelium that can result in disruption of blood flow [8].
Another research suggested additional mechanism of TAC involvement in alopecia through its main role in inhibiting T cell activation, which is considered a primary mechanism in the pathogenesis of alopecia [24].
Cyclosporine inhibits the nuclear factor on hair follicle stem cells which leads to an increase of the anagen phase of hair growth [25].
Frequently reported adverse effects inherent to CsA include gingivitis, gum hyperplasia and hirsutism.
A large prospective trial in recipients of renal transplantation demonstrated the occurrence of hirsutism in 8.3% of CsA-treated patients as a rescue therapy preceded by TAC [20].
Hypertrichosis affected 60% of Polish renal recipients in the study conducted by Imko-Walczuk et al. [9]. The immunosuppressive regimen for most of them was based on CsA. The connection between this disorder and CsA is well-documented [9]. The most significant hair growth is reported to occur in the period immediately following organ transplantation [26].
Other studies have also demonstrated an association between CsA and hypertrichosis. Bencini et al. conducted a study involving 67 kidney transplant recipients treated with cyclosporine and methylprednisolone, where hypertrichosis was the most common cutaneous adverse effect, reported by 60% of the patients [10]. In 1996, Busque et al. described 15 patients who were switched from CsA to TAC at their request, following the failure of cosmetic treatments for hypertrichosis, although the outcomes of this study remain unavailable [11]. In another study on mucocutaneous lesions in 54 kidney recipients on immunosuppression with prednisolone, azathioprine, and cyclosporine (mean daily doses: 10.2 mg, 68.6 mg, and 252 mg, respectively, with a mean cyclosporine concentration of 185 ng/ml), hypertrichosis was reported by 7 (12.9%) patients [12]. In 2009, a study among renal transplant recipients found that 23 patients experienced eyebrow and facial hypertrichosis, which resolved after conversion from CsA to TAC [13]. Furthermore, a 2011 prospective observational study of 346 kidney transplant recipients who switched from CsA to TAC revealed that 106 patients reported hypertrichosis, with 72% of them experiencing improvement or complete resolution after the conversion [14]. Similarly, a 2018 prospective observational study involving 266 stable kidney transplant recipients who switched from cyclosporine to tacrolimus highlighted that hypertrichosis was one of the reasons for conversion in 4 patients [15].
In 1983, Ringdén et al. conducted a study among 9 renal transplant recipients. Hirsutism was reported by 3 of them [16].
Also, in 2000, Higgins et al. conducted a study involving 19 renal recipients regarding conversion from TAC to CsA. One of these patients underwent re-conversion to TAC due to hirsutism caused by CsA [27].
Similarly, in 2005 Mohsin et al. conducted a study among 50 renal transplant recipients who had converted from CsA-based immunosuppression regimen to TAC. In a group of 12 patients, the reason for conversion was hirsutism [28].
Also, in 2005, Phillips et al. reported a paradoxical case of AA in two kidney-pancreas transplant recipients undergoing immunosuppressive therapy that included cyclosporine. The occurrence of AA in this population highlights the complexity of this immunological disease [29].
mTOR – sirolimus and everolimus
The mammalian target of rapamycin (mTOR) is a serine-threonine kinase involved in cell growth and proliferation. Medications that inhibit mTOR (SIR, EVE) possess not only immunosuppressive but also antiproliferative potential. They are weaker than calcineurin inhibitors (CsA and TAC) as far as immunosuppression is concerned but they are less nephrotoxic and are strong inhibitors of cell proliferation. Therefore, they are particularly suitable for patients with calcineurin inhibitors toxicity and also in transplant patients with non-melanoma skin cancer (NMSC).
In a specific study, twenty-eight liver transplant patients were treated with mTOR inhibitor – SIR/sirolimus or everolimus as rescue therapy after calcineurin inhibitor treatment. Some side-effects were noted but there was only one case of alopecia. The type of alopecia was not specified, and the researchers refrained from providing details on the characteristics of the hair loss. Their documentation only categorizes alopecia as one of the reported adverse effects [30].
Other drugs
Azathioprine (AZA) is the oldest immunosuppressive drug, used since the 1960s [31]. Azathioprine is the prodrug which is metabolized rapidly to 6-mercaptopurine (6-MP) and can be safely used even during pregnancy [32].
Seida et al. described a case report of a female who developed agranulocytosis and severe alopecia after initiation of AZA used as an alternative to MMF because of planning the pregnancy [33]. This patient had a homozygous polymorphism of NUDT15. Clinicians should remember to initiate treatment with low doses of AZA and consider interactions with allopurinol.
In 1993, Wagoner et al. conducted a study among 320 heart transplant recipients using AZA. Due to elevated liver enzymes, AZA was replaced with cyclophosphamide in 29 of these patients. One of these patients discontinued the therapy due to alopecia [34].
Mycophenolate mofetil (MMF) is the ester of 2-morpholinoethyl mycophenolate (MPA), which exerts cytostatic effects on T and B lymphocytes. Mycophenolic acid selectively and reversibly inhibits inosine monophosphate dehydrogenase, an enzyme involved in the synthesis of guanosine nucleotides necessary for DNA construction.
In the literature, there is no information regarding hair disorders caused by MMF used in renal transplant recipients. However, there is evidence of a lower risk of alopecia compared to intravenous cyclophosphamide for inducting remission in lupus nephritis (OR = 0.21; 95% CI: 0.12–0.36) [35].
In retrospective analysis of dermatological lesions in 183 kidney transplant recipients there is no information about hair problems associated with MMF. As a result, skin and mucosal diseases were reported in 173 (95.7%) of patients who were observed: viral lesions (50.81%); immunosuppression-related lesions (53.01%); benign tumours (16.39%); precancers /neoplastic lesions (15.3%); mycosis (14.21%); cutaneous xerosis (9.29%), dermatitis (8.74%). No cutaneous disease was evident only in 4.37% of cases. No hair problem was reported [36].
In the existing literature, there are no data elucidating the association between the type of transplantation and the specific manifestation of hair loss.
Discussion and conclusions
Gap in knowledge regarding alopecia
Long-term immunosuppressive therapy is a factor of various cutaneous side-effects. Infections and skin cancer are well-studied side-effects but other cutaneous drug reactions are rarely studied. The incidence of alopecia is probably underestimated by clinicians. It has been observed that assessment of alopecia is characterized by major discrepancy between patients and clinicians – 32% of transplant recipient-reported alopecia in comparison to 8.5% of physicians [19].
Retrospective analysis of Miotto et al. collecting data on skin reactions in a group of 532 SOTRs showed that diffuse non-scaring alopecia occurred in 23.1% of patients, more frequently in females (93.8%). Patients in this group were treated with prednisone and TAC [37].
In other long prospective analysis of renal transplant recipients, unusual hair growth was reported by 69.6% of patients. Average time since transplantation was about 5 years [38]. Alopecia, a side-effect that makes patients the most worried compared to other side-effects, is a pathology connected with many factors such as anemia and thyroid disorders and also autoimmune diseases, genetic factors, infections and stress [3, 39].
There is a lack of scientific studies confirming a decrease in the quality of life in patients experiencing hair loss after transplantation. Cosmetic side effects have psychological effects for patients, especially children and females [8]. The risk of alopecia in transplant patients should be discussed with recipients in order to avoid problems with non-compliance. It is important to explain that alopecia in this context can be reversible.
Importance of further research
Trichological problems, particularly alopecia, are complications observed in the group of patients undergoing immunosuppression after solid organ transplantation. While immunosuppression undeniably leads to various side-effects, its specific role in trichological problems remains incompletely understood, necessitating further studies. Research is warranted to investigate the prevalence of alopecia in transplant recipients, delineating patterns associated with specific organs and individual immunosuppressive medications. These studies aim to elucidate the etiopathogenesis of trichological complications post-transplantation and identify potential therapeutic interventions.
In the referenced studies, TAC was most frequently identified as a drug associated with hair loss [8, 18–24]. Other medications known to cause alopecia include AZA [32] and mTOR inhibitors [26]. There is no available information regarding alopecia in transplant recipients treated with MMF [35], whereas CsA has been linked to hypertrichosis [10]. However, one study showed that 30% of patients developed alopecia after CsA treatment, although this occurred significantly less frequently compared to TAC (56%) [19].
Methods
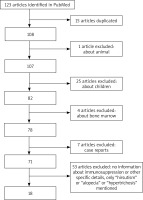
Table 1 present the search aimed to find data on hair disorders among immunosuppressed patients. The PubMed database was searched using different variations of primary keywords like: ”immunosuppression”, ”transplant recipients”, ”alopecia”, ” hirsutism” and ”hypertrichosis”. Inclusion criteria: article concerning incidence of immunosuppressive drugs among solid organ transplant recipients, adult patients, no animals, and no publishable data in the study. Articles not including information about immunosuppression or other specific details, and also articles about bone marrow transplantation, children, case reports and study about animals were excluded. The search was conducted in September 2024. The text above was based on the data included in the papers.
Table 1
Hair disorders among immunosuppresed patients
The flow diagram of this study is presented in Figure 1. A total of 123 potential studies and publications were identified. The selected publications covered the period from 1980 to 2024. Fifteen articles were excluded due to duplication. Additionally, articles were excluded for being focused on animals, children, or bone marrow transplantation. Studies with no information about immunosuppression or other specific details, or those only mentioning ”hirsutism”, ”alopecia” or ”hypertrichosis” were also excluded. The final set included 18 articles.