Introduction
Palpation with the assessment of tissue strain has long been an inevitable component of every medical examination. However, in the era of evidence-based medicine, this largely subjective examination has gradually begun to lose importance. The situation changed in the 1990s, when elastography – a type of ultrasonography suitable for quantification of tissue strain – entered clinical practice [1].
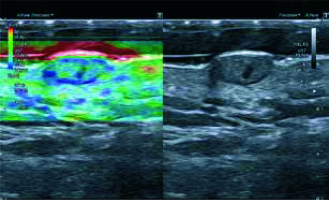
In simple terms, elastographic ultrasonography (EUS) can be considered a form of palpation whereby the examiner’s hand is replaced by the ultrasonographic transducer. However, the spectrum of tissues and organs that can be examined elastographically is considerably larger than the scope of conventional palpation [2]. Elastography provides information about the tissue strain or stiffness, which is independent of acoustic impedance and blood perfusion. Thus, the results of elastographic examination add to the evidence obtained during conventional B-mode ultrasonography and Doppler imaging (Figure 1). Elastography is based on the observation that various tissues differ in terms of their elasticity; therefore, this diagnostic modality can be used to distinguish between various tissues and, even more importantly, to monitor the course of local physiological and pathological processes [3]. Mechanical properties of the tissues are analyzed based on their deformation under applied force. The softer the tissue, the more it is deformed. Stiffer tissues undergo only minimal deformation and dislocation after applying pressure.
Types of elastography
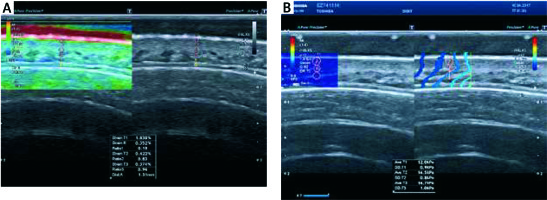
The first ultrasonographic devices suitable for real-time elastographic examination were marketed at the beginning of the 21st century. Currently available elastographic methods can be divided into static and dynamic (Figure 2).
In the case of static elastography, the pressure applied to examined tissue originates from external sources. Usually, the tissue is compressed with the ultrasonographic transducer, but sometimes the sources of the deforming force are the natural processes occurring within the tissues, such as pulsation of blood vessels and respiratory movements. The most popular method of static elastography is strain elastography (SE). During SE, elastographic images of examined tissue are acquired twice, prior to and after its controlled compression [4]. The images are digitally processed, and the results are presented as an elastogram depicting areas with various mechanical properties (stiff and elastic). Usually, the elastogram has the form of a color map, where tissues with various stiffness are designated by different colors. Typically, more elastic tissues are highlighted in red, and those with intermediate and low elasticity are highlighted in green and blue, respectively [5]. Alternatively, the tissues with high, intermediate and low elasticity can be presented in grayscale, as black, gray and white areas, respectively, or using a numerical scale [1]. The elastogram undergoes semiquantitative analysis by comparing it with a color-coded reference scale, or the strain ratio (SR) is calculated for a given tissue, as the ratio of its deformability to the deformability of an adjacent reference tissue [4].
The most commonly used dynamic elastographic method is shear wave elastography (SWE). In SWE, deformability is determined based on the velocity of a shear wave generated during the passage of an ultrasonographic beam across the tissue [6]. The shear wave velocity within softer tissues is lower, whereas the tissues with greater stiffness are characterized by higher values of this parameter. Consequently, shear wave velocity correlates positively with tissue strain. During the examination, the velocity of the shear wave generated by the ultrasonographic transducer is used to estimate the stiffness of the target tissue, expressed as Young’s modulus (E). The value of Young’s modulus for a given region of interest (ROI), expressed in KPa or m/s, is calculated by software integrated with the ultrasonographic device, based on the following formula: E = 3 pv2, where p is volumetric density of the tissue, and v is shear wave velocity.
Aside from the numeric value of Young’s modulus, the result of SWE can also be presented as a color-coded map applied to an ultrasonographic image of the tissue in grayscale. The results of SWE are more objective than those of SE, as they are 100% quantitative. Furthermore, the outcome is no longer biased by a subjective examiner-dependent component, i.e. manual compression of the tissue. As a result, SWE is characterized by less inter- and intra-observer variability than SE [6]. Due to the advantages mentioned above, SWE is gradually replacing SE in everyday clinical practice.
Principles of elastographic examination
Irrespective of the examined organ or anatomical region, elastographic imaging should always be carried out in line with the following guidelines prepared by experts from the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) [6]:
The examined structure should be in close proximity to the transducer (< 4 cm).
The structure should be nearly homogeneous.
When pressure is applied, there should be no slippage in the structure over deeper planes.
Pressure should be applied by a surface larger than the structure being examined.
No structures that damp compression, such as large blood vessels, should be present.
The structures being examined should be completely included within the ROI.
The direction of the compression force should be known.
The number of structures being examined should be limited.
Previous experiences with elastography in medicine
Due to the simplicity of the examination and the fact that it can be integrated with conventional ultrasonography, elastography is increasingly used in various medical disciplines. Elastographic ultrasonography is particularly widespread in oncology [7]; to date, elastography has been shown to be a valuable instrument for differential diagnosis of benign and malignant tumors of the breasts, prostate, uterine cervix, thyroid, liver, pancreas, salivary glands, musculoskeletal system and skin. Furthermore, EUS proved to be effective in identification of lymph node metastases [7]. However, the use of elastography is also expanding to other medical disciplines, for example obstetrics, where it serves as an adjunct test to evaluate cervical maturation in women at risk of preterm labor [8, 9], in hepatology, to assess the severity of liver cirrhosis [10], and in orthopedics, to visualize musculotendinous pathologies [4].
Skin as an object of elastographic examination
Theoretically, as a soft tissue, skin is a perfect candidate for elastographic examination. However, in practical terms, determination of skin strain by means of elastography faces numerous challenges. First, owing to the close proximity of the ultrasonographic transducer to the skin during the examination [11], the signal from deeper lying tissues may override the signal of skin detection. This refers in particular to the underlying tissues with higher values of Young’s modulus, such as muscles and bones [12]. Their close proximity to the skin may result in a decrease in the signal-to-noise ratio and lower reproducibility of elastographic parameters. Recently, also the thickness of subcutaneous fat has been identified as a significant contributor to lower repeatability and reproducibility of elastographic measurements [13].
Other factors that may potentially contribute to lesser accuracy of elastographic measurements include low thickness of the skin, its multilayered structure and variable orientation of collagen fibers [11, 14–16]. Skin, with an average thickness of only 1 mm, is composed of three layers with different elasticity: the epidermis, the papillary dermis and the reticular dermis with its adjacent subcutaneous tissue. The low thickness and considerable structural heterogeneity of the skin may significantly hinder selection of a representative ROI for elastographic examination [13, 17].
Some of the abovementioned limitations had already been overcome due to the use of high-frequency ultrasonographic transducers suitable for the examination of superficially located ROI with small diameters [18–20]. This contributes to better accuracy of SWE in the evaluation of skin strain. In a recent study of healthy volunteers, inter- and intra-observer intraclass correlation coefficients for Young’s modulus of the skin in various anatomical regions varied from moderate to excellent (0.62–0.91), and overall inter- and intra-observer repeatability equaled 0.908 and 0.885, respectively [20].
Elastographic parameters of normal skin and their determinants
To the best of our knowledge, except for a few studies [13, 17, 20, 21], elastographic parameters of normal skin have not been studied to date, and no published reference values for skin strain have been identified thus far. Moreover, most previous cutaneous elastography studies were relatively small observational studies or analyses of case series.
The strain of normal skin is known to vary across its layers. The dermis is characterized by higher strain than subcutaneous tissue [21]. Furthermore, the latter is not homogeneous in terms of its elastographic characteristics, due to the presence of stiffer connective tissue septa, and more elastic adipose tissue lobes, blood vessels and nerve fibers [21].
An elastographic study did not demonstrate statistically significant differences in SWE parameters of the skin covering bilateral structures (e.g. extremities) in 40 healthy volunteers [20]. The same study did not document significant differences in Young’s modulus for longitudinal and transverse skin cross-sections [20]. However, the evidence in this matter is still inconclusive, since in another smaller study of 18 patients, the values of Young’s modulus for transverse cross-sections of the forearm skin turned out to be significantly higher than for the longitudinal cross-sections [16].
A source of significant bias in cutaneous elastographic studies may be the plethora of variables that modulate the strain of normal skin. Elasticity of normal skin decreases with age due to qualitative and quantitative changes in the collagen fiber network. Furthermore, elastographic parameters of normal skin were shown to be influenced by anatomical region, sex, dexterity, physical activity, cigarette smoking, sun exposure and other environmental conditions, to mention a few [22].
Application of elastography in dermatology
Previous studies verifying elastography as a method to examine skin pathologies involved primarily cancer patients [23–27] and individuals with chronic systemic inflammation [28–36]. Most of these studies confirmed that elastography is an accurate method to identify and quantify proliferative and fibrotic processes taking place in the skin.
Benign and malignant skin conditions
Although benign lesions located in subcutaneous tissue can usually be accurately identified by B-mode ultrasonography, elastography may provide some additional useful data in doubtful cases. The authors of one study [37] used real-time elastography for differential diagnosis of 52 neck lesions. The study was semiquantitative; skin elasticity was graded on a numeric scale from 0 to 3, with 0 and 3 corresponding to the most elastic and stiffest areas, respectively. The accuracy of elastographic diagnosis was verified against the result of histopathological examination. The study demonstrated that lipomas were characterized by greater elasticity than other non-malignant skin tumors [37]. In another study, the same authors [38] used SWE to distinguish between benign and malignant neck tumors. Tissue stiffness within the malignant lesions was found to be approximately 8-fold higher than the stiffness within the benign tumors; this difference was statistically significant. When the cut-off value for Young’s modulus was set at 174.4 kPa, SWE provided 83.3% sensitivity and 97.5% specificity in differential diagnosis of benign and malignant lesions [38]. A study conducted by Korean authors demonstrated that inflamed epidermal cysts show lower elasticity than the unruptured cysts [39].
The results of previous studies imply that skin malignancies not only are stiffer than benign lesions, but are also less elastic than surrounding normal tissues [23]. The authors of one study [26] determined the strain ratio for 29 skin malignancies of epidermal origin (17 basal cell carcinomas and 12 squamous cell carcinomas) and 19 benign lesions in relation to surrounding tissues. Whereas the strain ratio for all malignant lesions was > 3.9, none of the benign tumors had a strain ratio greater than 3.0. These two cut-off values provided 100% sensitivity and specificity in differentiating between malignant and benign lesions [26]. Usefulness of elastography was also confirmed in the case of malignant melanoma of the skin. The authors of a pilot study on this subject [25] analyzed the strain of 42 melanomas using SE, and determined perfusion of the tumors with color Doppler imaging. Doppler ultrasonography confirmed the well-known fact that melanomas present with central ischemia and enhanced neoangiogenesis at the periphery [40, 41]. The SE added to these findings, demonstrating that the strain in malignant melanomas is significantly higher than in surrounding normal tissues [25]. Obviously, elastography will not replace diagnostic methods used routinely to evaluate proliferative skin lesions, such as biopsy. Nevertheless, combined with dermatoscopy, EUS may constitute a useful screening instrument at early stages of the diagnostic process.
Another potential application of elastography in oncology is identification of metastases in skin-draining lymph nodes [42]. Normal lymph nodes are characterized by relatively high elasticity, especially within the cortical part, which shows less strain than the capsule and hilum [19]. Usually, suspected lymph nodes are evaluated with a 4- or 5-point elastographic scale describing the proportion of stiff areas [43]. Similar to primary malignancies, lymph node metastases are stiffer than benign enlarged nodes [44]. However, this is not a universal phenomenon; for example, lymphomatous nodes tend to be more elastic than “typical” metastatic nodes and thus may be misinterpreted as inflammatory lesions [19]. This may limit the applicability of elastography in this indication; while metastatic nodes can be accurately identified based on their higher strain, less stiff nodes cannot be arbitrarily classified as benign on the basis of elastography alone [45]. Nevertheless, elastographic evaluation of lymph nodes has already found application in the diagnostics of solid tumors, including malignant melanoma. A few studies have demonstrated that addition of elastography to conventional B-mode ultrasonography and color Doppler imaging may increase their sensitivity in the detection of lymph node metastases up to 95%, with no effect on specificity [24, 46].
Chronic inflammation
Chronic inflammation alters the presentation of skin not only in conventional B-mode ultrasonography, high-frequency ultrasonography [47] and Doppler imaging, but also in elastography [48]. A study of 50 patients with skin abscesses demonstrated that SE may be used to detect stiff areas at the periphery of the lesions, which could not be visualized with conventional B-mode sonography [28]. Elastography was also used to monitor the response to topical corticosteroid therapy in 16 psoriatics [33]. Although the therapy contributed to a decrease in epidermal thickness and thickening of the dermis, these changes did not influence the elastographically determined stiffness of psoriatic plaques. This implies that practical application of elastography in psoriasis may be limited [33].
A large body of evidence regarding the usefulness of cutaneous elastography originates from studies of diseases associated with fibrotic and/or sclerotic processes, in particular systemic sclerosis. In the latter condition, stiffness of the skin is an important prognostic factor and a measure of disease severity, but available diagnostic methods are either subjective (palpation) or invasive (biopsy) [29]. Many authors have demonstrated that elastography is suitable for the assessment of skin stiffness in patients with systemic sclerosis. The only limitation of early SE-based studies in systemic sclerosis was low reproducibility of the results, especially for anatomical areas where the skin was located in the close vicinity of less elastic tissues, such as bones [29, 31]. However, this potential limitation can be overcome nowadays due to the application of modern SWE techniques. Regardless of the method, elastography provides accurate estimates of skin strain in other areas that are considered critical in patients with systemic sclerosis, for example, in the perioral region [32].
Practical applicability of elastography was also confirmed in the case of fibrotic and sclerotic lesions of other etiology, e.g. post-irradiation lesions, pressure ulcers and lipodermatosclerosis [30, 35, 36].
Application of cutaneous elastography in aesthetic medicine
Ultrasound examination, especially high-frequency, is a unique tool used in aesthetic dermatology to examine the side-effects of wrinkle fillers [49]. A growing body of evidence suggests that skin elastography may also find application in aesthetic medicine [19]. Elastographic parameters of the skin might serve as a measure of its true biological age, guiding the selection of tailored cosmetic-therapeutic procedures and parameters thereof. Moreover, elastography might be used to monitor recovery of the skin after various aesthetic procedures or even the outcome. However, all these applications of elastography necessitate the identification of reference ranges for facial skin elasticity. The latter issue, as well as verification of the usefulness of elastography in all the indications mentioned above, should constitute a subject of future research.
Conclusions
A growing body of evidence suggests that ultrasonographic elastography, a technique used to measure tissue stiffness, is suitable for the evaluation of skin pathologies, in particular benign or malignant masses and chronic fibrotic or sclerotic processes of either inflammatory or non-inflammatory origin. Due to the low thickness and multilayered structure of the skin, its elastographic evaluation requires a more accurate and less subjective method, such as SWE. Our knowledge about the elastographic parameters of normal skin is still sparse, which together with the lack of reference values for cutaneous stiffness constitutes a serious limitation to the use of elastography in aesthetic medicine.