Introduction
The family of vascular endothelial growth factors (VEGF) consists of five structurally similar forms: VEGF-A (the prototype, discovered first and called generally VEGF), VEGF-B, VEGF-C, VEGF-D and placenta growth factor (PGF). VEGF is a homodimeric glycoprotein, weight 46 kDa, its gene is located on 6p21.3. VEGF variants with a different number of amino-acids are produced in human cells by alternative splicing (for VEGF-A, VEGF-B and PGF) and processing (VEGF-A, VEGF-C and VEGF-D). Among them, VEGF121 and VEGF165 are generated by macrophages, platelets, neutrophils and epithelial cells as soluble and freely diffusible forms, whereas VEGF189 and VEGF206 are associated with cells’ surface. VEGF acts biologically by receptors tyrosine kinases (RTKs – VEGFR-1, VEGFR-2, VEGFR-3), which differ considerably in signalling properties and are expressed by activated macrophages, endothelial cells or epithelial cells [1–3]. VEGF is the main regulator of vascular development and vessels function in health and disease. VEGF seems to be a key regulator of physiological angiogenesis during post-natal and skeletal growth, reproductive functions, embryogenesis with endothelial cells’ growth, menstrual cycle and wound healing [4, 5]. VEGF has also been implicated in pathological angiogenesis in several malignancies, e.g. in tumour growth and the formation of metastasis, age-related macular degeneration, autoimmune diseases, allergic and metabolic disorders, cardio-vascular and dermatological diseases [6, 7]. VEGF has been considered as one of the molecules important in asthma, lung remodelling and activation of cells involved in the inflammatory process, which include basophils. Basophils synthesize IL-4 and IL-13, what suggests that they participate in IgE synthesis and Th2 polarization. These cells are also able to produce pro-angiogenic molecules, like IL-3 and VEGF. Despite their small numbers in peripheral blood, basophils are involved in disorders with immune background, such as allergies, autoimmune and infectious diseases, cancers and immunodeficiency [8–10]. Detection of activated basophils by indication of CD203c marker on the membrane using the flow cytometry has been shown to be a useful tool. Up-regulation of CD203c on basophils’ surface is the result of FcεRI-mediated reaction and plays a role in cytokine-mediated activation of these cells [11, 12].
Aim
The aim of the present study was to reveal the possible relationship between CD203c expression as a marker of basophils activation by VEGF in asthmatics – in subgroups with and without irreversible bronchoconstriction. The influence of the genetic factor as the del18/ins -2549 -2567 polymorphism in the promoter region of the VEGF’s gene was also considered.
Material and methods
Study group
The study involved 122 individuals (80 women) aged from 20 to 70 years, of whom 40 participants from the control group (26 women) had normal pulmonary function and did not present any allergies. In a group of 82 patients (54 women) aged from 23 to 69 years, the diagnosis of asthma was established according to recommendations from the Global Initiative for Asthma (GINA 2011). The degree of asthma severity ranged from sporadic to severe persistent. Considering the bronchodilation test, patients with asthma were divided into two subgroups: patients without irreversible bronchoconstriction (40 patients; 29 women; aged from 23 to 69 years) and patients with irreversible bronchoconstriction (42 patients; 25 women; aged from 24 to 69 years). After genotyping of VEGF polymorphism, the results provide three genotype classifications: homozygous mutation (ins/ins), heterozygous mutation (ins/del) and no mutation or the so-called wild genotype (del/del). Therefore, participants from all the examined group were divided into two phenotypic categories: no mutation – del (del/del) and mutation – ins (ins/ins or ins/del). The protocol for our study was approved by the Ethics Committee of the Wroclaw Medical University, Poland (No. KB 68/2011). Characteristics of the study population are shown in Table 1.
Table 1
The population data
Bronchodilation test
The pulmonary function test with the bronchodilation test, defining among others forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) values, were established using MasterScope CT Spirometer (Erich Jaeger GmbH, Wurzburg, Germany). Each parameter was estimated three times and showed as a percentage of predicted values; the best scores were used for further analysis. In asthmatics, the bronchodilation test was performed after inhalation with 5 mg of salbutamol (SteriNeb Salamol; Teva Pharmaceuticals, Warsaw, Poland) administered with the jet nebulizer (Model 4650-U, Devilbiss, Heston, UK). The post-bronchodilator values of FEV1/FVC < 0.7 and FEV1 < 0.8 predicted were taken as a criterion of irreversible bronchoconstriction.
Basophils activation
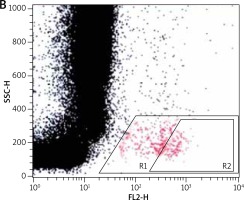
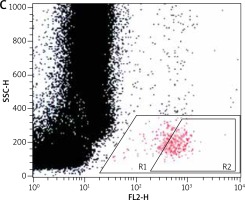
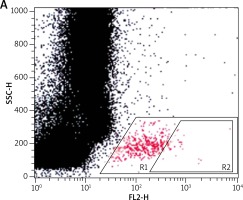
For the flow cytometric method, following samples were used: sample without VEGF as a non-stimulated negative control (patient background – Pb), sample with fMLP (N-Formylmethionyl-leucyl-phenylalanine) in concentration of 10–6 M as a positive control (Pc) and two samples containing VEGF in concentration of 250 ng/ml and 500 ng/ml. All samples contained 100 µl blood taken to the 4.5 ml tubes with lithium heparin (Saerstedt AG & Co., Nümbrecht, Germany) and 100 µl RPMI-1640 Medium (Institute of Immunology and Experimental Therapy, Wroclaw, Poland), fMLP (Institute of Immunology and Experimental Therapy, Wroclaw, Poland) or VEGF (BD Biosciences Pharmingen, San Diego, USA). Samples were incubated in the atmosphere supplemented with 5% CO2 at 37°C for 60 min (Incubator ASSAB, Stockholm, Sweden). After incubation, 20 µl of edentate disodium EDTA (BD Biosciences Pharmingen, San Diego, USA) was added and samples were centrifuged at 1600 rev/min for 10 min. Then, the supernatant was removed, 100 µl of PBS (Institute of Immunology and Experimental Therapy, Wroclaw, Poland) with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, USA) and 10 µl of anti-CD203c (Immunotech S.A.S., Marseille, France) were added and incubated in the darkness in 25°C for 30 min. After incubation, 2 µl fluid for the cells’ lysis (BD Biosciences Pharmingen, San Diego, USA) was added and after 10 min at room temperature, the samples were centrifuged at 1600 rev/min for 5 min. Afterwards, the supernatant was removed and for each sample 3 ml of PBS was added and centrifuged 5 min at room temperature at 1600 rev/min. To preserve the cells, 200 µl of PBS with 1.5% paraformaldehyde (Sigma-Aldrich, St. Louis, USA) was added. From each examined sample, 105 cells were collected using a FACScan flow cytometer (Becton Dickinson, San Diego, USA) and expression of antigen CD203c on basophils surface was used for analysis of their activity. In total population of basophils (gate R1), the percentage of active cells was identified (gate R2) – it is shown in Figures 1–3. The above method used for basophils activation test was coincident with that described by Kahlert et al. [13] with own modification (two samples containing VEGF in selected experimentally concentration of 250 ng/ml and 500 ng/ml).
Figure 1
Population of basophils (gate R1) with the active cells (gate R2) in a non-stimulated negative control sample
Genotyping of VEGF’s polymorphism
DNA was isolated from blood lymphocytes using a DNA isolation kit (QiAmp DNA Blood Mini kit, Syngen Biotech, Wroclaw, Poland). All patients from asthmatics and controls groups were genotyped by polymerase chain reaction (PCR) according to the method by Lachheb et al. [14] with the own modification. The pair of primers (F: 5’-cctggagcgttttggttaaa-3’ and R: 5’-atataggaagcagctggaa-3’) was used for the verification of the addition or the loss of 18 base pairs (18 bp) within the promoter region in VEGF gene at a position -2549 -2567. The concentration of the isolated DNA and its purity was identified by spectrophotometer (NanoDrop ThermoScientific, Thermo Fisher Scientific, Wilmington, USA). The total volume of the PCR was 25 µl containing 100 ng of genomic DNA, 0.5 mmol/l of nucleotide, 3 pmol of suitable starter, 1 x Taq Buffer and 0.5 units of Taq-DNA polymerase (Taq DNA Polimeraza E2500-02 – 5000u, EURx, Gdansk, Poland); the final MgCl2 level was 4 mmol/l. The PCR comprised an initial denaturation step (95ºC for 15 min), then 35 cycles (95ºC for 30 s), primer annealing (54ºC for 30 s and 72ºC for 30 s) and a final extension step (72ºC for 10 min). The PCR products were analysed using agarose gel electrophoresis followed by ethidium bromide staining and ultraviolet visualization.
Results
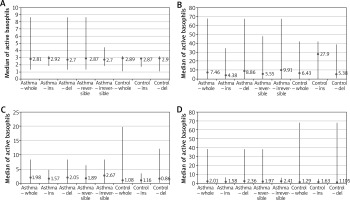
In the non-stimulated negative sample (Pb), the percentage of activated basophils was at a similar level in all tested persons. In controls, the median of activated cells was 2.89% (min. 1.06%; max. 3.2%) – in controls with del phenotype it was 2.9% (min. 1.06%; max. 3.2%) and in controls with ins phenotype it was 2.87% (min. 1.71%; max. 2.9%) and this difference was statistically significant (p = 0.03). In asthmatics, the median of activated basophils was 2.81% (min. 1.31%; max. 8.63%) – in a subgroup with reversible bronchoconstriction it was 2.87% (min. 1.31%; max. 8.63%) and in a subgroup with irreversible bronchoconstriction it was 2.7% (min. 1.83%; max. 4.42%). Asthmatics with del phenotype and ins phenotype had a similar level of activated basophils (Me = 2.7% (min. 1.31%; max. 8.63%) vs Me = 2.92% (min. 1.83%; max. 3.21%)) and this difference was not statistically significant (p = 0.18).
After stimulation of fMLP (positive control), the percentage of activated CD203c+ basophils was higher than in a non-stimulated negative sample. In controls, the median of these cells was 6.43% (min. 0.0; max. 42.02%) – in controls with del phenotype it was 5.38% (min. 0.93%; max. 38.49%) and in controls with ins phenotype it was 27.9% (min. 0.0; max. 42.02%) and this difference was statistically significant (p = 0.03). In asthmatics, the median of activated CD203c+ basophils was 7.46% (min. 0.26%; max. 67.33%) – in a subgroup with reversible bronchoconstriction it was 5.55% (min. 0.45%; max. 47.54%) and in a subgroup with irreversible bronchoconstriction it was 9.91% (min. 0.26%; max. 67.33%). Asthmatics with del phenotype and ins phenotype had a different level of basophils (Me = 8.86% (min. 0.56%; max. 67.33%) vs Me = 4.38% (min. 0.26%; max. 34.2%)) and it was statistically significant (p = 0.02).
After addition of VEGF in concentration 250 ng/ml, the median of activated basophils in asthmatics rose to a mean value of 1.98% (min. 0.0; max. 8.31%) and was higher than in controls (1.08% (min. 0.0; max. 19.8%)). In a subgroup with reversible bronchoconstriction the median of activated basophils was 1.89% (min. 0.0; max. 6.34%) and in a subgroup with irreversible bronchoconstriction it was 2.67% (min. 0.0; max. 8.31%). After considering presence of del/del genotype, there was a significant difference (p = 0.0007) between percentage of activated CD203c+ basophils in asthmatics and controls (Me = 2.05% (min. 0.0; max. 8.31%) vs Me = 0.86% (min. 0.0; max. 19.8%)).
After increase in the VEGF concentration to 500 ng/ml, the median of activated basophils in controls rose to a mean value of 1.29% (min. 0.0; max. 67.96%) and was higher in persons with del phenotype (1.11% (min. 0.0; max. 67.96%)) than with ins phenotype (1.63% (min. 0.0; max. 5.13%)). In asthmatics, this median was 2.01% (min. 0.0; max. 38.36%) – with reversible bronchoconstriction it was 1.97% (min. 0.25%; max. 38.36%) and with irreversible bronchoconstriction it was 2.41% (min. 0.0; max. 6.3%). After considering the genetic factor, there was a significant difference (p = 0.02) between percentage of activated CD203c+ basophils in asthmatics del/del genotype vs ins/ins and ins/del genotype (Me = 2.36% (min. 0.04%; max. 38.36%) vs Me = 1.58% (min. 0.0; max. 6.49%)). The data are shown in Figures 4 A–D.
Discussion
Vascular endothelial growth factor, described by Senger et al. [15] and then by Ferrara and Henzel [16], is an important angiogenic factor. It is an essential molecule for the growth of new blood and lymph vessels in adult life, in health and during disorders, likewise allergic. Authors have observed a relationship between circulating VEGF and intensity of neovascularization and inflammation in airways in asthmatics [17–19]. Increased concentration of VEGF was present in asthmatics in comparison with healthy controls in the sample of induced-sputum, in the bronchoalveolar lavage or in bronchial biopsies [20, 21]. VEGF increases the number of vessels in the lamina propria of airways in asthmatics. It also affects the airways hyperactivity and thickening of the bronchial wall during lung remodelling [22, 23].
The angiogenic process requires an interaction among specific growth factors (e.g. VEGF) and cell types (e.g. basophils, mast cells). Available studies indicate that basophils can synthesize and release VEGF and are susceptible to VEGF-activation. De Paulis et al. [24] observed a high expression of mRNA for VEGF and VEGF-R in circulating basophils and intracellular presence of VEGF in basophils located in airways. Afterwards, the VEGF release after basophils activation and increasing chemotaxis was shown. Immunologically activated human basophils selectively express not only isoforms of VEGF-A and VEGF-B but also release angiopoietin-1 that activates mast cells and in this way might participate in the complex network involving inflammatory angiogenesis [25]. Observations by Lichtenstein et al. [26] confirmed the role of basophils in asthma – there was a positive correlation between the number of basophils obtained from bronchoalveolar lavage and the bronchial hyperreactivity. Furthermore, migration of activated basophils to the airways was observed during asthma exacerbation which was associated with an increased number of cells in sputum. The basophils activation test (BAT) with the flow cytometry method is a reliable tool for monitoring basophils activation by detecting expression of markers on the membrane, e.g. CD63 or CD203c [27–29]. Moreover, the possible relationship of the VEGF gene polymorphism with the VEGF-activated basophils in asthmatics has not been specified yet. Therefore, the purpose of the present study was to analyse the activity of basophils in all groups of patients, using the flow cytometric methods with anti-CD203c and with samples containing fMLP (positive control), the increasing concentrations of VEGF (250 ng/ml and 500 ng/ml) and in the sample of unstimulated cells (negative control). The influence of a genetic factor (polymorphism del18/ins -2549 -2567 in the VEGF-promoter region) was also considered.
Having basophils activity expressed as cells with the receptor CD203c on the surface, the median percentage of the activated basophils in the unstimulated cells sample was found to be similar in the control group and in the groups with asthma, regardless of the genotype del18/ins or the severity of the bronchoconstriction. After stimulation of basophils with fMLP, the statistically significant difference in the activity of these cells was revealed between the control group and in patients with asthma, also having considered presence of del18/ins genotype. Analysis of the activated basophils after stimulation with increasing concentrations of VEGF showed a similar percentage of activated cells in each group of patients. The statistically significant difference was seen in patients with asthma and different genotypes del18 vs ins of the VEGF gene as well as between the group of patients with asthma with del18 genotype and the control group with del18 genotype.
Obtained data indicate that the stimulation of basophils with VEGF enhances the activity of these cells. Additionally, in patients with del18 genotype both in the control and asthmatic group, the activity of basophils after VEGF stimulation remains higher, compared with the sample of unstimulated cells.
Our study has potentially significant drawbacks – the use of only one marker (CD203c) to determine the degree of basophil activation, experimental selection of only two VEGF concentrations and lack of occurrence of inhalation allergy that may affect the BAT results. With the promising obtained results, it is possible to extend our work in the future by other markers of basophil activation (e.g. CD164, CD63) and other simulating concentrations of VEGF or/and other cytokines.
Conclusions
We present our findings to draw attention to the potential role of the VEGF and its genetic variants in the basophil activation. Undoubtedly, the pathomechanism of the airways remodelling in asthma is complex. Our results seem to form a logical sequence and may indicate another pathway of the process which has not been subjected to consideration yet. Polymorphism del18 in the promoter region -2549 -2567 of the VEGF gene is related to the increased activation of basophils by VEGF and, because of that, with the greater predisposition to release mediators from these cells. Both processes contribute to the development of remodelling. The above hypothesis is worth a further investigation to be conducted on a larger sample of patients.