Introduction
Venous leg ulcers (VLUs) belong to the category of the most common chronic wounds of the lower limb. They constitute the most severe complication of chronic venous insufficiency (CVI). Approximately 2% of the adult population and 5% of senior citizens (over 65 years of age) develop active or healed ulceration [1–6]. The main etiological factors of VLUs are chronic venous hypertension and venous stasis, which is why compression therapy remains the mainstay of treatment for the majority of patients. Treatment outcomes depend on multiple prognostic factors, such as duration and wound size, recurrence rate and anatomical location of venous pathology [7–9]. The majority of ulcers are slow-healing wounds with a high recurrence rate of 70% and a 60% risk of becoming chronic [10, 11]. Particularly troublesome are the so-called non-healing wounds, which will not heal for many years. Approximately 20% of ulcers are reported to remain active for about 2 years and 8–10% remain active for 5 years. Ulcers active for over 10 years have also been reported [3, 4, 6, 8, 9]. Chronic ulcers are characterized by permanent inflammation and high activity of proteolytic enzymes [12, 13]. Histological analyses among the patients with CVI have shown the infiltration of skin and subcutaneous tissue pericapillary space by multiple inflammatory cells, cytokines (interleukin 1α and 1β – IL-1α and IL-1β, tumor necrosis factor α – TNF-α), platelet adhesion activators, lysosomal proteolytic enzymes (mainly elastase), toxic oxygen metabolites and free radicals among others. In comparison with the control group, excessive catabolism of connective tissue and improper arrangement of collagen fibres have been observed among patients with VLUs [13–18]. In vitro studies on skin fibroblast culture have demonstrated that these pathologies may be the consequence of prolonged inflammation since typical inflammatory mediators decreased the synthesis of procollagen I and collagen and disrupted its development and structure. Similar observations have been conducted in fibroblasts cultured in fibrin gel [13, 19, 20]. Still, collagen is an essential structural protein, important in maintaining the mechanical function of the skin as well as wound healing. It constitutes more than 50% of all the proteins taking part in this process [13]. The exposure of collagen fibrils in damaged tissues sets in motion a chain of cellular and biochemical reactions which initiate haemostasis and subsequent proliferation. Collagen constitutes a mechanical framework for developing structures. Its synthesis, growth and arrangement have a vital influence on the final properties and resistance of scars. In addition to its scaffold function, collagen is a critical signalling molecule in the extracellular matrix [20]. The above-mentioned properties of collagen have long been used in the production of dressing materials, such as haemostatic dressings (collagen supports haemostasis) [21], biological dressings (in the treatment of chronic wounds and burns that are hard-to-heal), bioactive dressings (collagen acts in synergy with other elements of the extracellular matrix, for instance, fibronectin, elastin, glycosaminoglycan) [13, 22–26] and antibacterial dressings (collagen utilized as a vehicle for an antibiotic to facilitate its penetration into deep structures of infected tissues) [27, 28]. Collagen dressings usually do not produce side effects but their effects are limited to the wound bed. However, patients with VLUs also suffer from accompanying tissue trophic lesions (such as hemosiderosis, lipodermatosclerosis, atrophie blanche) and skin complications [5, 6, 29–32]. Many of them complain of persistent itch, excessive dryness and skin scaling. Some struggle with eczema – stasis dermatitis or contact (allergic) dermatitis, which increases the risk of microtrauma, infection and formation of new ulcers [20–34]. Intensive skin care, promoting its integrity and protective function may improve the effects of complex therapy of VLUs. Wounds start to heal from the edges, that is, the proliferation of granulation tissue and epidermis takes place as a result of the division of the surrounding healthy and intact cells. By contrast, the so-called aging cells show disrupted proliferative potential and they do not differentiate properly. That is why any intervention which aims to improve skin condition and promote healing should not be underestimated. We have assumed that topical application of collagen will reduce inflammation surrounding the wound and enable faster healing of ulcers. We used collagen in the form of gel intended to be applied directly on the skin around the wound, instead of the previously used dressing. So far fish collagen has been primarily used in the beauty industry as an ingredient in creams and skin care products in order to improve the overall condition of the skin on the face and body. To the best of our knowledge, no research into the influence of external use of fish collagen on the healing process of VLUs and the condition of periwound skin has been conducted.
Aim
The aim of this study was to assess the efficacy of fish skin collagen and its impact on the process of healing of VLUs. We assessed the progress of wound healing by planimetry and infrared thermography (IRT).
Material and methods
In this study we have exogenous collagen from the skin of freshwater fish. Collagen was obtained from farmed form of Silver carp skin (Collagen Active Science Sp. z o.o., Poznan, Poland). The manufacturer had controlled biological contamination of the material. It was found that the gel was free of microbiological contamination. Also all fatty acid was removed during the manufacturing process. No heavy metals were found. The collagen in the gel was in its native form, the denaturation temperature was found at 33.4°C (by viscometric method). The gel contained water, lactic acid, preservative (Rocoal MD) and collagen of about 1% concentration. The electrophoretic studies showed the presence of 200 kDa collagen β-chains and 130 kDa collagen α-chains. The gel was not applied directly to the wound and was a cosmetic product that met the standards for use on the skin of infants and around the eyes.
Ethics statement
The project received ethical clearance as a prerequisite of approval for funding from the National Centre of Research and Development as a part of Applied Research Projects (No. NCBiR, PBS3/B7/28/2015). The study protocol was approved by the local bioethics committee of the Nicolaus Copernicus University in Torun, and Ludwik Rydygier Collegium Medicum in Bydgoszcz (No. KB 69/2015) and conducted in accordance with the Helsinki Declaration of 1975. All enrolled patients signed a written informed consent form. Patients’ data were managed in accordance with the Polish Data Protection Act [35].
Participants
This 12-week randomized single-centre study included 100 adults (> 18 years) with chronic venous ulcers. Recruitment occurred at a highly specialized national centre for chronic wound healing, in the period from 2016 to 2019. The study classification criteria included the presence of a leg ulcer (area between 5 and 50 cm2), CVI, as proven by scanning of the lower extremity blood vessels (duplex scan), a duration of ulceration > 3 months, an ankle brachial index (ABI) of 0.9–1.3, and a lack of clinical symptoms of infection. The exclusion criteria were ulcerations of mixed aetiology other than venous or undiagnosed and coexisting lower limb disorders. Eligible patients were randomized to either tropocollagen gel treatment (group A) or placebo alone (group B) using computer generated random numbers. Several patients withdrew during the study or missed follow-up visits after the wound healed. We included 97 patients in the final analysis (A, n = 48; B, n = 49). Baseline characteristics of patients are shown in Table 1.
Table 1
Baseline characteristics of the patients studied
| Characteristic | Group A (n = 48) | Group B (n = 49) | ||
|---|---|---|---|---|
| Mean ± SD (range) | Median | Mean ± SD (range) | Median | |
| Age [years] | 64.4 ±11.6 (35–88) | 64.5 | 62.4 ±13.00 (39–87) | 64 |
| Gender*: | ||||
| Female | ||||
| Male | ||||
| Duration of CVI [years] | 17.8 ±12.7 (1–50) | 15 | 16.4 ±13.5 (1–52) | 12.5 |
| Duration of VLU [months] | 71.3 ±103.8 (3–440) | 20 | 39.2 ±60.7 (2–370) | 19 |
| BMI | 30.7 ±7.3 (19.4–58.9) | 30 | 30.7 ±6.6 (20.1–58.9) | 30.5 |
| Initial wound size [cm2] | 18.1 ±14.8 (5–50) | 11.3 | 15.1 ±14.2 (5–50) | 8.7 |
| ABI right | 1.09 ±0.11 (0.88–1.33) | 1.06 | 1.13 ±0.14 (0.8–1.5) | 1.15 |
| ABI left | 1.08 ±0.12 (0.83–1.4) | 1.06 | 1.11 ±0.15 (0.72–1.5) | 1.10 |
Interventions
All groups received standard wound care twice weekly for 12 weeks or until healing was complete. Standard treatment for VLUs included 2nd class compression therapy with short-stretch bandages Matopress and cotton wool pads Matosoft Natural (TZMO Matopat, Torun, Poland), used for skin protection purposes. Wound hygiene procedures [36] included cleansing the wound and periwound skin, debridement, renewing the wound edges, using the dressing appropriate to the healing phase. Moreover, patients in group A had tropocollagen gel applied on periwound skin, while patients in group B received placebo. We applied 5 cm3 of the formulation at a time (that is, 10 pumps of the dispenser). The gel was applied on clean skin within 2 cm from wound edges. The gel was massaged into the skin moistened with saline solution (approximately 10 ml) using circular finger motions (we wore disposable gloves). Halfway through the application process, we would again moisten the skin with saline (0.9% solution) and gently rub in the remaining gel that had not been absorbed fully. The total application time was equal to 15 min. The application took place twice a day for 12 weeks (even if the wound had completely healed). The nurse applied the gel during appointments at the clinic, while patients applied it on their own at home. The patients had been instructed on how to take care of the wound earlier and each bottle of the gel came with a written instruction of the application process.
Ulcer area
We assessed the dynamics of wound healing by planimetry and infrared thermography every 2 weeks during a 12-week study. Moreover, at week 24 from the beginning of the study the patients were invited to a single follow-up appointment, during which all the measurements were taken once again. Wound area calculations were performed using a Visitrak digital wound measuring device (Visitrak Digital: Digital-Pad, Smith & Nephew, Austria). The primary endpoint, complete wound healing, was defined as a 100% reduction in wound area. Secondary endpoints included reduction in wound area by 60%, change in wound area (in cm2 and as a percent change from baseline), ulcer healing rate assessment for small (< 10 cm2) and large (≥ 10 cm2) ulcers (in cm2 per week), and the percentage change (from baseline) in wound bed tissue composition, including epithelial tissue, granulation tissue, fibrin and sloughy tissue [36, 37].
Infrared thermography (IRT)
The severity of periwound inflammation was assessed by means of infrared thermography. We used mobile, high-resolution infrared camera (FLIR Systems, Wilsonville, OR, USA; model T650sc; 640 × 480 pixels). We took the photos in projection perpendicular to the anterior surface of the leg (the photo included the extremities from the knees to the feet). All photos were automatically saved in IRT and digital versions. Image analysis was conducted using FLIR Tools+ as software. The software automatically calculated the temperature in the area specified by the researcher. We analysed the mean, median and extreme values of temperature (minimum and maximum) on both lower extremities. According to the guidelines [38] for thermographic analysis, we compared the periwound temperature of the limb with an ulcer present to the contralateral area on the non-ulcerated limb. This constituted a point of reference.
During the process of photo taking we followed the guidelines of the International Academy of Clinical Thermology (IACT) [38, 39]. We ensured optimal conditions in the research facility and prepared patients for examinations. After removing all clothes, bandages, and dressings from the legs, we placed the patient in the correct position for the photo to be taken. Acclimatization lasted at least 15 min. If the wound was contaminated and required debridement, the acclimatization time was measured once the wound care procedures had ended. The collagen gel was applied after taking temperature. In this manner, we eliminated the direct influence of skin preparation and skin massage on the temperature of the extremities, which would have compromised important measurement data. The results of initial examination and detailed thermographic evaluation of the healing process in patients with VLUs were discussed in another publication [40].
Moreover, all patients were asked to write down their subjective perceptions such as pain, discomfort, drainage, burning sensation or other adverse reactions. Adverse reactions were classified as “present” or “absent”; their severity was assessed on a 5-point scale (1 – absent, 2 – mild, 3 – moderate, 4 – quite severe, 5 – very severe).
Results
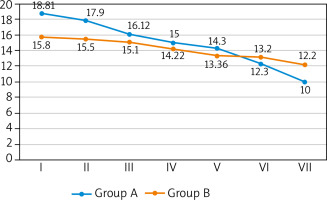
A greater number of complete ulcer healing was observed in the study group. By week 12, there were minor differences between both groups (14 (29.2%) vs. 11 (22.4%)). During week 24, more pronounced differences were observed during the follow-up appointment (25 (52.1%) vs. 18 (36.7%)). At that time, patients in the study group showed an increase in the number of ulcers healed by 15.4%. The percentage of healed wound surface was greater in the study group but the difference became noticeable only in week 24 (Me: 100% vs. 88.2%). Wound healing progress was observed in both groups but the ulcers in the study group healed faster. Faster healing time in the study group was observed in both big as well as small ulcers. Big ulcers were characterized by a faster healing rate in weeks 6, 12 and 24 of the observation. Statistically significant differences between groups occurred only in week 24 (Me: 0.19 vs. 0.10 cm2/week; p = 0.005). The healing rate of small ulcers until week 6 was comparable in both groups whereas during week 12 the healing rate increased in the study group, with the differences just at the limit of statistical significance (Me: 0.15 vs. 0.14 cm2/week; p < 0.001). By week 24 all small ulcers healed, both in the study group as well as the control group. We observed far greater growth in the epidermis, greater growth in the granulation tissue and decrease in the fibrin/necrosis in the wound in subsequent measurements in the study group. A detailed characteristics and healing process parameters in both groups were presented in Table 2. Changes in the surface of wounds in the consecutive weeks of observation were shown in Figure 1.
Table 2
Wound healing parameters at selected time intervals
| Variable | Units | Group A | Group B | P-value | SMD |
|---|---|---|---|---|---|
| The number (%) of completely healed ulcers*: | |||||
| n | 48 | 49 | |||
| After 6 weeks | n (%) | 4 (8.3) | 3 (6.1) | 0.768 | 0.101 |
| After 12 weeks | n (%) | 14 (29.2) | 11 (22.4) | 0.644 | 0.108 |
| After 24 weeks | n (%) | 25 (52.1) | 18 (36.7) | 0.155 | 0.313 |
| The number (%) of ulcers healed in 60%*: | |||||
| n | 48 | 49 | |||
| After 6 weeks | n (%) | 13 (27.0) | 12 (24.5) | 0.445 | 0.208 |
| After 12 weeks | n (%) | 28 (60.9) | 25 (54.3) | 0.673 | 0.132 |
| After 24 weeks | n (%) | 33 (71.7) | 27 (61.4) | 0.372 | 0.221 |
| Healing rate – big ulcers > 10 cm2**: | |||||
| n | 25 | 19 | |||
| After 6 weeks | cm2/week, Me (SD) | 1.08 (0.86) | 0.81 (1.49) | 0.467 | 0.217 |
| After 12 weeks | cm2/week, Me [IQR] | 0.75 [0.02, 1.18] | 0.44 [–0.22, 1.33] | 0.656 | 0.142 |
| After 24 weeks | cm2/week, Me [IQR] | 0.19 [0.09, 0.54] | 0.10 [–0.07, 0.65] | 0.542 | 0.005 |
| Healing rate – small ulcers > 10 cm2**: | |||||
| n | 23 | 30 | |||
| After 6 weeks | cm2/week, Me [IQR] | 0.75 [0.57, 0.92] | 0.65 [0.43, 0.90] | 0.285 | 0.354 |
| After 12 weeks | cm2/week, Me (SD) | 0.15 (0.27) | 0.14 (0.20) | 0.824 | 0.063 |
| After 24 weeks | cm2/week, Me [IQR] | 0.00 [0.00, 0.04] | 0.00 [0.00, 0.09] | 0.867 | 0.287 |
| The median percent of the healed ulcer area*: | |||||
| After 6 weeks | (%) Me [IQR] | 48.61 [19.36, 85.03] | 50.59 [16.30, 78.81] | 0.858 | 0.061 |
| After 12 weeks | (%) Me [IQR] | 70.43 [38.43, 100.00] | 67.79 [33.55, 98.15] | 0.606 | 0.135 |
| After 24 weeks | (%) Me [IQR] | 100.00 [53.56, 100.00] | 88.23 [45.22, 100] | 0.235 | 0.278 |
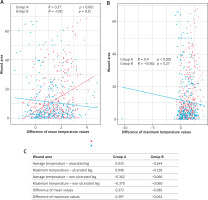
The thermographic analysis showed statistically significant reduction in periwound inflammation among patients from the study group. We observed significant changes of all temperature values (that is, mean, median, maximum) from the periwound skin area with the exception of the minimum value. Mean and maximum temperature of the ulcerated limb decreased as time passed. Furthermore, in the study group the temperature of the ulcerated limb became equal to the temperature of the contralateral limb. Statistically significant reduction in mean as well as maximum values of limb temperature occurred only in the study group (Tx: R = 0.37, p < 0.001; Tmax: R = 0.40, p < 0.001). Such relationship was not observed in the control group (Figure 2).
Figure 2
Analysis of the dependence of average and maximum temperature values on changes in the wound area. A – Graph of correlation between the difference of mean temperatures and changes in the wound area. B – Graph of correlation between the difference of maximum temperatures and changes in the wound area. C – Characteristics of the correlation between temperature values and the healing process in the studied groups
In both groups patients reported local reactions from the beginning of the observation. During the treatment, 2 patients from the study group and 1 patient from the control group complained of itch, rash, redness and skin maceration. These symptoms subsided when the dressing was changed to an absorbent one and an extra absorbent layer was used (2 patients). One patient required dermatological consultation. After 2 weeks of eliminating the allergen (most likely soap), the symptoms subsided. As for the skin symptoms reported at the beginning of the therapy, they subsided in 62.5% of patients from the study group and 35.8% of patients from the control group in the course of the study or once it ended (Table 3).
Table 3
Local skin symptoms/reactions
| Variable | Unit | Group A | Group B | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Skin reactions* | 24 (50.0) | 9 (18.7) | 28 (57.1) | 18 (36.7) | |
| Itch | n (%) | 11 (22.9) | 2 (4.2) | 12 (24.5) | 5 (10.2) |
| Rash/allergy | n (%) | 5 (10.4) | 1 (2.1) | 4 (8.2) | 2 (4.1) |
| Redness | n (%) | 9 (18.7) | 3 (6.3) | 11 (22.4) | 7 14.3) |
| Burning sensation | n (%) | 5 (10.4) | 2 (4.2) | 6 (12.2) | 3(6.1) |
| Skin maceration | n (%) | 6 (12.5) | 3 (6.3) | 4 (8.2) | 3 (6.1) |
| Pain** | n (%) | 8 (16.7) | 3 (6.3) | 7 (14.3) | 3 (6.1) |
Discussion
This study shows that a 12-week treatment with fish collagen gel improves the healing of venous leg ulcers and the condition of periwound skin. Previous studies have confirmed the effectiveness of collagen dressing in the treatment of chronic wounds [13, 16, 19, 21–28]. The effectiveness of dressings containing porcine small intestine submucosa (SIS) [41–43] and bovine collagen in combination with oxidized regenerated cellulose (ORC) [44–46] has been well documented in randomized controlled trial (RCT). Such dressings were used in the treatment of chronic wounds of various aetiologies, including VLUs, mixed leg ulcers and DFUs. During the course of 12-week therapy, 37–55% of wounds treated with collagen dressings healed, as compared to 28–34% of wounds treated in a standard manner. Good results were also obtained in several RCT assessing the acellular human dermal matrix [47, 48]. After 12-week therapy, healing was observed in 70–85% of cases, as compared to 45–50% of DFUs; following 16 weeks, the results were 70% and 46%, respectively. The results indicated the advantage of collagen but they were inconclusive. Diverse research methods, different clinical characteristics of wounds and small sample size made a reliable comparison impossible.
It is often stressed that some of the collagen production methods may be expensive and cost-ineffective, which results in the limited use of collagen biomaterials in the treatment of chronic wounds [13]. In our study we used collagen from the skin of freshwater fish (silver carp), which is inexpensive, easily available and characterized by good physicochemical properties [13, 24, 33, 34, 49]. Fish collagen has a relatively low molecular weight (less intermolecular crosslinking), which makes it possible to obtain a fully biologically active molecule in the form of helix. Tropocollagen is highly biocompatible, nonallergenic and, as opposed to the land-based animal collagen, free from the risks of animal diseases and pathogens [13, 24]. We conducted a pharmacoeconomic analysis which showed that the treatment with fish collagen is cheaper than the standard treatment of VLUs. The cost of obtaining one effect in the collagen treatment technology was 33% lower than in the previous treatment technology [50].
We were the first to use fish collagen in the gel form on periwound skin, and not in the wound bed. In the preliminary analysis conducted in the group of 59 patients with VLUs we assessed the effect of the formulation on periwound skin and its ability to penetrate the corneal layer of the epidermis [49]. Electrical impedance measurements showed the improvement in the overall skin condition [49] while cutometry showed the improvement in skin elasticity and firmness after the use of collagen. In the placebo group, skin improvement was not significant [49, 51, 52]. It is worth noting that the positive effect of collagen on the skin was noticeable only from week 8 of the therapy [49]. This is also true in terms of the impact the collagen had on wound healing. By week 6, the healing rate was similar in both groups and it was only between week 8–10 that the dynamics of ulcer healing in the study group improved. We obtained a greater number of complete healing (by 6.8% in week 12 and 15.4% in week 24), higher percentage of epidermal growth and granulation tissue as well as decrease in severity of subjective (such as pain, itch, burning sensation) and objective (redness, rash) skin symptoms. We may infer that the collagen gel applied on periwound skin first improved the overall skin condition and only secondarily enhanced the process of healing. In vitro studies have demonstrated that the topical use of collagen modulates chronic wound environment, mainly through the optimization of inflammation. The stabilization of the protease level and pro-inflammatory cytokines, whose abundance usually inhibits chronic wound healing [53–55], has been observed among others. We did not assess the physicochemical properties of the microenvironment of the wound and its surroundings, but compared the temperatures of the ulcerated limb to the contralateral area of the non-ulcerated limb. The difference of the maximum temperatures between an inflamed and a healthy area may equal approximately 1.5–2.2°C and even more in case of infection [56]. Reducing this difference is a good predictive factor, thus IRT assessment may prove helpful in the prognosis for wound healing [40, 56]. It has been shown that higher temperature within the wound bed and its surroundings is associated with a several-times increased risk of delayed healing, and even wound deterioration. The authors have emphasized that the rise in wound temperature may be associated with critical colonization, infection and other factors which slow down healing. In this study, the temperature difference between the limbs decreased in the consecutive measurements. This value was significantly correlated with the progress of wound healing only in the study group. In the control group, the temperature differences did not decrease significantly and periwound inflammation persisted for a longer period of time.
An unquestioned strength of this study is the 12-week follow-up period. To our knowledge, this is the first study to assess the progress of VLU treatment over such a long period of time using infrared thermography. Unfortunately, we have not assessed whether and for how long the effect on the skin and local inflammation persists after discontinuing the use of collagen. We believe that the use of collagen should be considered until the wound is healed.
Conclusions
The results described in this study suggest that the use of fish collagen in gel on the periwound skin is beneficial. Several weeks of therapy with the use of collagen have reduced inflammation and accompanying unpleasant sensations as well as improved healing of venous ulcers provided it is used in combination with standard wound bed debridement, hygiene and compression therapy of chronic venous insufficiency.