A 33-year-old man was presented with flat-topped lesions distributed over the limbs and trunk accompanied with occasional pruritus for nearly 8 years. No other cutaneous or mucous lesions were detected. He denied any significant medical problems or family history. On examination, there were discrete, grouped, flesh-coloured, flat-topped papules or vesicles measuring 1 to 2 mm in diameter distributed over the limbs and lower abdomen (Figure 1 A). Histopathological examination showed the focal degeneration of the basal layer with obvious separation of epidermis immediately beneath well-circumscribed lymphohistiocytic cells infiltrating like “ball-in-claw” rete ridges (Figure 1 B). To further determine the histologic origin of vesicles, immunohistochemistry of CD31/CD34 (blood vessel marker) and D2-40 (lymphatic vessel marker) was performed. But no positive staining of vesicles was found (Figures 1 C, D). Based on clinical and histopathological findings, we further confirmed the final diagnosis of vesicular lichen nitidus (VLN). However, the lesions were hardly improved after topical corticosteroids.
Figure 1
Clinical and histopathological images of the lesion from the patient. A – Primary lesion on the abdomen. B – Histopathological examination showed the focal degeneration of the basal layer with obvious separation of epidermis immediately beneath well-circumscribed lymphohistiocytic cells infiltrating like “ball-in-claw” rete ridges. C, D – Immunohistochemistry of CD31/CD34 and D2-40
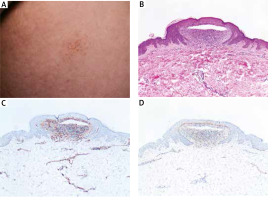
The lichen nitidus (LN) is a rare inflammatory cutaneous condition with characteristic histopathology first mentioned in 1907. Two features were distinguished in the LN. The typical clinical lesions of LN commonly present as discrete, flat-topped, grouped, skin-coloured papules on the limbs and abdomen. Histopathologically, a cluster of lymphohistiocytic infiltration was confined to dermal papillae, which consist of “claw clutching a ball” pattern. Immunoglobulins could not be detected in lesions of typical LN through immunofluorescence [1]. In addition, LN presents a fairly distinctive dermoscopic character. A shiny elevated surface without dermatoglyphic patterns was presented in non-polarizing dermoscopy, while ill-margin hypopigmentation with linear vessels and diffuse erythema were detected in polarizing dermoscopy [2]. Clinically, a variety of different LN manifestations have been reported, including generalized, linear, perforating, haemorrhagic and vesicular variants [3–6]. In particular, LN presented as blister lesions is rather uncommon [5]. The vesicular variation was first mentioned by Jetton and Eby in 1971, which extended the spectrum of LN. Up to date, only two cases have been reported. Both of the cases reported involved young male adults with typical lesions and more glistening dome-shaped vesicles, which were similar to our patient’s. And our case is the second report of vesicular lichen nitidus in the Chinese population. We further excluded endogenous vasogenic and lymphangitic pathogenesis leading to the formation of vesicles by immunohistochemistry of CD31/CD34 and D2-40. Therefore, exaggeration of liquefaction degeneration in the basal layer rather than vasogenic/lymphangitic obstacle would be the pathomechanism in vesicular lichen nitidus (VLN).
The clinical appearances of lichen dermatitis were thought to be a varying degree of spectrum disorders. And whether LN shifted to lichen planus (LP) as a cutaneous progression was still a matter of debate. Previous cases reported LP occurring subsequent or coexisting in LN [7]. Similarly, a vesicular phenotype of LP has also been reported, which is bullous lichen planus (BLP). Some associations may exist between VLN and BLP. Both in VLN and BLP, the histopathological examination showed sub-epidermal blisters infiltrating dense inflammatory cells in the papillary layer of the skin, providing further support for this debate.
Although the exact mechanism of LN is still unknown, it may be associated with familial, Down syndrome, Crohn’s disease, tuberculosis or immune disease [8–11]. Some therapeutic approaches have been effective on the lesions, like topical steroids, enoxaparin sodium, tacrolimus ointment, antituberculous drug, acitretin, and phototherapy. However, the clinical treatment response is still unpredictable.
LN presented as vesicular eruption is rare. And in histopathological terms, blister formation may suggest that lesions tend to exacerbate in LN. Here, we reported this case to further reveal the features of VLN.








