Introduction
There is growing evidence that some plant components play a role in reducing the incidence of lifestyle diseases, often causing chronic ulcers. Since the risk of chronic ulceration is partly age-related and the European population is greying, it is worthwhile to investigate this feature of plants carefully and in more detail. A major input will have to come from the aetiology of the very diverse types of ulceration as well as from epidemiological studies.
Since ancient times, flax (Linum usitatissimum) has been known to be a source of oil and fibres and has been cultivated as a dual-purpose plant for a long time. Flax seed oil is valued primarily for its nutritional properties as well as for the associated health benefits. Although its fatty acid composition is most often noted, with oil content of 35–50%, whole flax seed is additionally comprised of approximately 20% of protein, 28% of dietary fibre, and 6–8% of polysaccharide mucilage along with an array of phytochemicals. In addition, constituents exist within the oil such as vanillin, tannins, p-coumaric, chlorogenic, caffeic, ferulic acid, coniferyl and syringic aldehyde from phenylpropanoid pathway and plastochromanol-8, tocopherol and lutein of terpenoid pathway [1]. Those compounds have been shown to exhibit pharmacological activity. Flax seed oil contains linoleic acid (LA) and linolenic acid (ALA) as its major omega-6 and omega-3 polyunsaturated fatty acids (PUFA), respectively. These fatty acids comprise the most desirable contents of the oil.
The myriad of benefits reported to be attributable to flax seed constituents include anticancer (breast and prostate), anti-inflammatory, anti-thrombotic properties and additionally help to increase general metabolic rates [2, 3] and promote the burning of fat [4]. Flax may also lessen the severity of diabetes by stabilizing blood-sugar levels. There is some support for the use of flax seed as a laxative due to its dietary fibre content though excessive consumption without liquid can result in intestinal blockage.
The seedcake, which is rich in antioxidants, might be used in the pharmaceutical and cosmetic industries. Phytochemicals that have been identified in flax seedcakes mainly consist of lignans, isoprenoids, phenolic acids, flavonoids and cyanogenic glucosides. All these compounds, apart from cyanogenic glucosides, are known to have antioxidant properties or inhibitory activity against carcinogen induced tumours (flax contains up to 800 times more lignans than other plant foods).
Flax fibres are flexible, lustrous and soft. Moreover, they absorb humidity and are allergen-free. Besides polymers (cellulose, lignin, pectin, hemicellulose) flax fibres contain numbers of bioactive molecules including: 4-hydroxy-benzoic acid, vanillin, ferulic acid, syringic acid and aldehyde, sterols (campesterol, β-sitosterol), and unsaturated fatty acids. Cannabidiol (CBD) and polyhydroxybutyrate (PHB) have been also found to be present in flax fibres and shives. The presence of these chemicals makes flax fibres extremely useful in the medicine.
Additionally recently, some research has been carried out to improve the quality of flax oil (higher stability, optimal w6/w3 ratio) and fibres (supplemented with antioxidants, analgesic compounds, new polymer) and make them even more suitable for the biomedical industry [5–8]. Innovative flax oil and fibre-containing products have been developed with potential applications in the medical field. The main strategy was to make use of genetically modified flax fibres with unusual and unique properties. Therefore nowadays, genetically modified flax appeared to be not only a dual- but rather multi-purpose plant and its exploitation is not restricted to the production of linen fibres and oil.
All these might suggest the usefulness of flax as a source of new raw materials for generating innovative products for medicine. One such product is proposed in this work for chronic wound healing.
Chronic wounds include venous ulcers, ischemic wounds (mostly of atherosclerotic origin), diabetic foot syndrome, and decubitus (trophy) ulcers. Chronic non-healing ulcers are a critical problem in clinical practice. Slow healing, difficulty in providing proper healing support/treatment methods and suffering of the patient are great challenges for modern medicine. Venous ulceration represents the most prevalent form of non-healing wounds and these problematic wounds significantly impair the quality of life, and require a significant amount of healthcare resources for their treatment [9, 10]. Although researchers and clinicians are developing novel therapeutic approaches to promote healing, the goal is still beyond reach. It is estimated that about 1% of the total health care costs in the Western world is likely to be used for management of chronic leg ulcers [11].
In this study we prepared and investigated wound dressings, based on genetically modified flax fibre, which except a high quantity of natural phenolics, sterols, cannabidiol and unsaturated fatty acids, contains PHB [8, 12], and oil and seedcake preparations based on transgenic flax plants overproducing phenylpropanoids [13]. The PHB is embedded in the fibre and thus it changes its structure, which cannot be mimicked by simply adding external PHB to cellulose fibre [12]. Biochemical properties of the new flax dressings are evidenced here as well as their effect on wound healing.
Material and methods
Plant raw products
The fibres were extracted from flax plants (Linum usitatissimum cultivar Nike) transformed with three bacterial (Ralstonia eutropha) genes overproducing polyhydroxybutyrate in vascular boundless (M plant) [5]. The seeds of the plant (Linum usitatissimum cultivar Linola) transformant (W plant) was the source of the oil and seedcake extracts which serve as the basis of polyunsaturated fatty acids and the myriad of compounds of phenylpropanoid and terpenoid pathways, respectively. These plants were transformed with three cDNAs encoding chalcone synthase (CHS), dihydroflavonol synthase (DFR), and chalcone isomerase (CHI) [7, 14, 15]. Transgenic plants were field trialled, and the 6th generation has been used as a source of fibres and seeds. The details on plant transformation, selection and transgenic plant analysis were described previously [5, 15]. The transgenic plants grown in the field were harvested after 4 months. After that the seeds were collected and fibres extracted from the straw. The oil extraction was conducted by cold pressing of the seeds. The press cakes (seedcake) were utilized for extraction of bioactive phytochemicals.
Wound dressing constituents
Linen fabric
The lignocellulose fibre was extracted from the stem of the flax plant by dew retting. This process separates the straw or bark from the fibre and is carried out by spreading the flax in the field and exposing it to atmosphere for 3–4 weeks. After the retting is complete, the flax passes through the mechanical devices that break the stem, separate the outer fibres from the phloem and remove the broken barks. This process ultimately releases flax fibres from the stem. Small pieces of the bark, broken in this process, are called shives. Dry spinning of fibres produced yarns which was used for making coarse fabric. It should be emphasized that during the entire fabric preparation process no chemicals were used, and thus the material retains all bioactive compounds, however, it has a slight elasticity.
Linen dressing
The dressing for wound coverage was prepared from raw yarn using the standard weaving method. The linear mass of the warp and weft was 68TEX. An appropriate quantity of the linen dressing was sterilized by autoclaving at 120°C for 20 min. The size of the dressing for wound treatment was 10 × 10 cm. Where indicated, the linen dressing for the wound treatment was covered with 2 ml of the sterile seedcake extract or with 2 ml of oil emulsion.
Preparation of oil emulsion
Flax oil emulsion was prepared according to the published protocol [16]. Briefly, soybean lecithin (Lipoid S75 from Lipoid, Ludwigshafen, Germany) and Tween 80 (Sigma-Aldrich) were mixed with flax oil from transgenic flax plants overproducing phenylpropanoid compounds (oil composition was carefully described by Zuk et al. [17] and Hasiewicz-Derkacz et al. [1] and additionally controlled in every batch of used oil), and subsequently an aqueous phase containing glycerol (Sigma-Aldrich) was added, mixed and sonicated with a Microson ultrasonic cell disruptor for 10 min at 4 W, room temperature, followed by filtration through sterile Acrodisc (Gelman Sciences, Ann Arbor, MI) 0.45-μm filters.
Preparation of seedcake extracts
Transgenic flax plants overexpressing three genes coding for enzymes of the phenylpropanoid pathway [14] were used for seedcake extract preparation as described previously [7] and have been thoroughly examined, among others, to determine their bactericidal properties [13]. The extracts were sterilized by filtration through 0.45 μm Acrodisc filter (Gelman Sciences, Ann Arbor, MI) or by autoclaving at 120°C for 20 min.
Development of integrated wound dressings
The fabric was moistened with either a physiological salt solution (Lenplast BIS 1) or oil emulsion (Lenplast BIS 2), or a seedcake extract (Lenplast BIS 3), and used for stage 1, stage 2 or stage 3, respectively. In each case, the dressings were sterilized by autoclaving (120°C for 20 min) prior to use. The procedure was standardized for all samples and stability (quantitative and qualitative invariance) of bioactive compounds was checked after the sterilization and storage process (up to 12 months).
Biochemical composition of dressings
Phenolics extraction
1 g of the wound dressing (Lenplast BIS 1, 2 or 3) was ground in an MM200 Retsch mill to a fine powder and extracted three times with methanol. After merging the extracts and evaporating under a vacuum, they were re-suspended in 1 ml of methanol (fraction I – free phenolics). The remaining material was hydrolysed in 2 M NaOH for 24 h at room temperature to release ester-bound phenolic compounds. Extracts were adjusted to pH 3, extracted with ethyl acetate three times to separate the phenolics, then the ethyl fraction was dried under a vacuum and re-suspended in 1 ml of methanol (fraction II – cell-wall bound phenolics).
LC-MS analysis of low-molecular phenolics
The components were analysed using the Acquity UPLC system (Waters) with photodiode array detector (PDA) and QTOF mass detector as described previously [1]. The identities of components were determined based on their retention times, UV and mass spectra comparison to authentic standards (Sigma-Aldrich, USA).
Terpenophenol extraction and analysis
The linen dressing (15 g) was extracted three times with 50 ml of chloroform, the solutions were pooled, and after drying in a pure nitrogen atmosphere, the matter was re-dissolved in 500 μl of ethanol. The extract was filtered through 0.25 μm Acrodisc and the concentration of cannabidiol in the extracts was measured. The analysis was performed with a Waters Acquity ultra performance liquid chromatograph with a PDA and mass detector as described previously [18]. Detection and integration of the cannabidiol peak was performed at 230 nm in comparison to the compound standard (Sigma-Aldrich, 98.5% purity).
Sterol extraction and analysis
Dressing samples (200 mg) were cut and milled to achieve good sample fragmentation. The sample was first extracted three times with 5 ml of chlorophorm : methanol (1 : 1) mixture. Then, the extract was evaporated under nitrogen and re-extracted with 30 μl of hexane containing 1 mg/ml 5α-cholestane as an internal standard. The sample was placed in a glass test tube with a Teflon seal screw cap. 2 ml of 2 M KOH in anhydrous methanol was added to each sample and mixed well. Closed tubes were then heated for 45 min at 70°C, vigorously shaken every 10 min. 1 ml of distilled water, 1.5 ml of hexane and 0.5 ml of 96% ethanol were added to the cooled samples, and the whole samples were mixed thoroughly for 5 min. After separation into layers, the hexane layer was collected and placed into a new glass tube with a Teflon cap, and the extraction procedure was repeated with another 1.5 ml of hexane. The extracts were pooled and evaporated under nitrogen (25°C) to dryness. Trimethylsilyl derivatives of sterols were prepared according to the method described by Styrczewska et al. [18, 19].
Fatty acid extraction and analysis
Dressing samples (3 g) were cut into small pieces and extracted twice with a MetOH/chloroform (2 : 1) mixture for 40 min with constant shaking. Combined extracts were filtered, supplemented with 1/4 volume of distilled water, shaken and left overnight at 4°C to separate the layers. On the next day, chloroform layers were collected and supplemented with methanol and 0.9% NaCl to a final ratio of MetOH : chloroform : NaCl of 8 : 4 : 3, mixed well and left at 4°C overnight to separate layers. Collected chloroform layers were evaporated under nitrogen to obtain the final volume of 10 ml. Then, the samples were again mixed with methanol and 0.9% NaCl (the same final ratio as before), shaken well and centrifuged to separate the layers. Cleaned chloroform layers were finally evaporated to obtain a final volume of 500 µl. Chloroform extracts (100 µl) were placed in glass tubes with a Teflon sealed screw cap and supplemented with 500 µg of pentadecanoic acid as an internal standard. 1 ml of 0.5 M KOH in anhydrous methanol was added to each sample, which was then shaken well and incubated for 30 min at 70°C. After cooling, 1 ml of 1.25 M HCl in anhydrous methanol was added, and the samples were mixed and incubated at 70°C for 30 min. Samples were cooled and 1 ml of hexane and 3 ml of saturated NaCl were added to each. Samples were then mixed well for 5 min and the hexane fractions containing fatty acid methyl esters (FAME) were collected into new Eppendorf tubes. Extraction was repeated by adding an additional 1 ml of hexane, and the collected hexane fractions were stored at 4°C until measurement (on the next day at the latest). FAME analysis was carried out using an Agilent 7890A gas chromatograph with a FID detector as described previously [8]. Series of fatty acid standards (Sigma-Aldrich) were analysed to determine the retention times for each compound.
Measurement of bioactive compounds in vitro release from dressings
The integrated wound dressing (about 3.83–4.0 g – 10 cm2) was incubated in buffered saline solution (10 ml, PBS) for 12 h at 37°C. After incubation, the solution was adjusted to pH 3 and extracted 3 times with ethyl acetate. The ethyl acetate fraction was dried under a vacuum, re-suspended in 1 ml of methanol and analysed in Ultra Performance Liquid Chromatography (UPLC; Waters Corp., Milford, USA).
For measuring the release of 3-hydroxybutyrate from dressing, a commercially available assay (β-hydroxybutyrate Assay Kit, Sigma-Aldrich) was used. In this assay, the concentration of β-hydroxybutyrate is determined by an enzymatic reaction wherein the product is determined colorimetrically (at 450 nm).
Determination of bioactive compounds migration through the semipermeable membrane – an in vitro test
Vertical Franz diffusion cells made of amber glass (FDC 400; Crown Glass, Somerville, NJ, USA) equipped with semi-permeable membrane (Dialysis Tubing Visking, Medicell International Ltd., London, UK) were used to test the release of most representative bioactive compounds (ferulic acid, phenolic acid glucosides and SDG) across the membrane. The compounds were prepared in the form of a gel in 3% carbopol. Membrane was hydrated in a methanol-water mixture (7/3; v/v) for 24 h before mounting in a Franz diffusion cell. Phosphate buffered saline (pH 7.4, 32 ±0.5°C) stirring at 700 rpm in receptor chamber, provide sagging conditions. Samples of 3 g gel (95% linseed extract) were placed in the donor chamber in direct contact with the membrane (0.64 cm2), and then 500 μl of the sample was collected with the syringe from the receptor compartment at predetermined times. Samples were analysed by liquid chromatography (Ultra Performance Liquid Chromatography (UPLC); Waters Corp., Milford, USA).
DPPH scavenging assay
In order to determine the antioxidant properties of the flax dressings, the DPPH method was used [20]. 1 g of the dressing was extracted with methanol, and the extract was supplemented with 0.1 mM DPPH radical in methanol, mixed thoroughly, incubated for 30 min at 37°C, and absorbance of the solution was measured at 515 nm. The radical scavenging activity was calculated using the equation: % DPPH scavenging = (1 – Asample/Acontrol) × 100%, where Asample = absorbance of extract, Acontrol = absorbance of DPPH solution.
TBARS assay
1 g of the flax wound dressing was milled in an MM200 Retsch mill and extracted three times with 1 ml of methanol in an ultrasonic bath for 20 min. The extract was centrifuged at 18,000 rpm for 10 min at room temperature. The combined supernatants were vacuum dried and re-suspended in 1 ml of methanol. The level of thiobarbituric acid-reactive substances (TBARS) was measured according to the published protocol [21].
Effect of dressing’ compounds on fibroblasts growth, migration
Human skin fibroblast culture conditions
Normal human dermal fibroblasts (NHDF) (PromoCell, Germany) were cultivated in sterile conditions in 5% CO2 atmosphere and at 37°C temperature. NHDF were grown in DMEM medium containing 1.5 g/l glucose (LONZA), 10% foetal calf serum (Biological Industries), 100 U/ml penicillin, and 100 g/ml streptomycin (LONZA), until 90% confluence and then trypsinized (0.05% trypsin/1 mM EDTA in calcium- and magnesium-free PBS (LONZA)). Trypsin was inhibited by addition of fresh medium in a 5 : 1 volume ratio; cells were centrifuged at 800 ×g for 5 min and further plated to obtain 7 to 10 times area dilution.
Cell morphology observation and basic proliferation tests
For the basic proliferation test, NHDF cells were cultivated in 24-well plates for 24 h and after the fresh medium was added, incubated with increasing concentrations of the methanolic extract from Lenplast BIS 1, Lenplast BIS 2, and Lenplast BIS 3. Cell morphology was assessed for compliance with the line specification. The morphology of the cells were visualized after 24 h of incubation, using a transmitted light phase contrast microscope equipped with 10× and 100× objectives (Axiovert 40 CFL, ZEISS). The laboratory has an algorithm for cell culture evaluation, which includes macroscopic and microscopic evaluation as well as culture identity assessment, i.e. whether the cells meet the features included in the specification. The most frequently observed morphology altering effects are the appearance of numerous vacuoles around the nucleus (over 20% cytoplasm), membrane fragmentation and detachment of cells. We call all these morphology changes the cytopathic effects.
After that, the MTT reagent (Sigma, USA) was added to make a final concentration of 0.4 mg/ml and incubated for 4 h. Produced formazan crystals were dissolved in 0.5 ml of DMSO (Sigma, USA) for 30 min and measured at 540 nm in an Asys UVM340 Microplate Reader (Biochrom, UK). The experiment was performed in four biological replicates (each time on fibroblasts from separate passages (culture bottles) and with the use of ethanol extracts from different samples of dressings (another batch of fabric, emulsion, seedcakes preparation and separate sterilization)).
In vitro wound scratch assay
Wound healing properties were evaluated using the in vitro scratch assay [22], which measures the expansion of a cell population on surfaces. NHDF were cultivated in a 24-well plate for about 48 h, until nearly confluent monolayers were obtained. Next, the monolayer was linearly disrupted with a sterile 10 µl plastic pipette tip. The cellular debris was removed by washing the culture with PBS, and then fresh medium was added, containing 0.5% foetal calf serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Then, the flax dressing extracts were added. Non-treated cells served as a control, but also ethanol samples were analysed. The experiment was performed in four biological replicates.
NHDF treatment for gene expression analysis
For the treatment, the NHDF cells (PromoCell, Germany) were grown in six-well plates for 24 h. After fresh medium had been added, the inflammatory state was induced with LPS (100 ng/ml final concentration in the culture) in PBS. The incubation lasted 6 h, before the dressing extract and control samples were added and incubated for 24 h. An ethanol negative control and CBD and β-sitosterol standard positive controls were also included pure CBD in final concentrations corresponding to flax preparations (0.1 µg/ml), and β-sitosterol 1.82 µg/ml. The ethanol concentration was 0.1%. The cells were harvested after 24 h of treatment and directly used for RNA isolation.
To determine the activity of the histone acetyltransferase/histone deacetylase genes, NHDF cells were grown in a cell culture dish (100 mm) for 24 h. After adding fresh medium, the incubation was continued for 6 h, and after the 3-hydroxybutyrate monomer at the final concentration of 50, 75 and 200 µM in PBS was added. Afterwards, the flax fabric (containing 50 µmol of PHB) was added and incubation was continued for 24 h at 37°C. The cells were harvested after 24 h of treatment and directly used for total RNA isolation.
Total RNA was isolated from freshly harvested cells after the treatments
Total RNA was isolated from freshly harvested cells after the treatments using an RNeasy Plus Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. To exclude genomic DNA contamination, 2 μg of each RNA sample was treated with DNase I (Fermentas, Lithuania) and directly used as a template for cDNA synthesis. cDNAs were synthesized using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Real-Time PCR reactions were carried out according to SYBR Green Master Mix (Applied Biosystems, USA) manufacturer’s instructions; the primer sequences are presented in Supplementary Table 1. Obtained data were analysed using ΔCt methodology for calculation of the parameter RQ (relative quantification).
Table 1
The percentage of total (at the start point of storage) content of bioactive compounds after 3, 6 and 12 months of storage at 4–8°C
| Compound name | Seedcake extract | Lenplast BIS 3 | |||||
|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | 3 months | 6 months | 12 months | ||
| Ferulic acid | 100%** | 60.27%** | 50.74%** | 100%** | 89.48%* | 82.42%* | |
| Coumaric acid | 86.42%* | 78.19 %** | 45.26%** | 96.71%** | 84.36%* | 75.16%* | |
| Ferulic acid glucoside | 85.16%** | 50.74%** | 30.54%* | 100%** | 98.88%* | 94.64%* | |
| Coumaric acid glucoside | 97.81%** | 97.32%* | 84.39%** | 100%** | 100%** | 99.11%** | |
| SDG | 65.27%** | 42.05%* | 28.21%* | 94.18%** | 91,87%* | 83.87%* | |
Antimicrobial activity indication
The bacterial strains (Staphylococcus aureus strain ATCC 6538, Bacillus subtilis strain ATCC 6633) used as indicators for antimicrobial activity testing were obtained from the in-house culture collection of the Microbiology Department (University of Wroclaw, Poland). Bacteria were grown at 37ºC in Luria-Bertani (LB) medium under shaking conditions. Diluted (100 fold in LB) overnight cultures of all tested bacteria strains (150 μl) were incubated in 96-well plates at 37°C, shaken at 600 rpm with previously prepared Lenplast BIS 1, 2, 3 extract. The volumes contained the standardized proportions of 50 to 500 µM of vanillin. OD600 was monitored for 12 h, at 1 h interval in Asys UVM340 Microplate Reader (Biochrom, UK).
Clinical trial
Patients
The study group included 22 patients (12 males and 11 females, mean age of 65 ±8 years), who suffered from chronic non-healing ulcerations of venous origin for more than 12 months (mean duration of ulceration of 9.2 ±8 years). Preliminary clinical and instrumental screening was performed with 823 consecutive patients having lower extremity ulcerations in a dermatology outpatient setting of the Wroclaw Military Hospital over a 2-year period. Apart from venous ulcerations, this represents decubitus ulcers, extremities’ ulcers due to atherosclerosis obliterans, diabetic foot, ulcers due to skin cancers and other skin changes. From 823 patients with ulcers who have applied to the outpatient clinic, only 156 (19%) people had a purely venous ulcer cause. Of these, 22 people were qualified for the study. There were mostly patients who had been diagnosed with venous ulcers and treated using local and general pharmacological therapy of wounds (different kinds of traditional gauze and bioactive dressings available on the market, compression therapy, antibiotics, analgesic and anticoagulant therapy) for years. Prescribed therapy, although consistent with current medical guidelines, had not resulted either in healing or in any improvement of the wound. A few patients presented for the first time for consultation to clarify etiopathogenesis of leg ulcers. The group of subjects with ulcerations of the lower extremities invited to the study included those who fulfilled the criteria for participation in the programme. In all the cases, the wounds were located on lower limbs. Inclusion criteria: recruitment into the programme was limited to adult patients (> 18 years old) with stable ulceration of venous origin lasting 1 year or more, despite careful, systematic patient’s and doctor’s care in accordance with the standards (including compression therapy), with no macro- or microscopic evidence of bacterial infections (patients recruited to the programme had not received antibiotics) and with disqualification of surgical treatment of ulcers after angiosurgery consultation. All eligible patients who were invited to the project were asked for consent. The baseline characteristics of the entire patient population referred to the project are summarized in Supplementary Table 2. Exclusion criteria included: ulcers of aetiology other than venous, indicated more effective treatments for ulcers than the use of dressings (i.e. surgery), a bacterial infection requiring antibiotic therapy, the lack of cooperation from the patient, and the lack of consent to participate in the project.
Table 2
Content of phenolic compounds [ng] and PHB/3HB released from 1 g of fabric after 48 h incubation in buffered pH = 6.8 saline solution (PBS)
Treatment of patients
Routine laboratory tests were performed on all the patients during their first and last visits and are depicted in Supplementary Table 3. Peripheral blood was collected in the morning after an overnight fast. Arterial pathology was excluded by clinical evaluation, ABI measurement (Sonodop 4000 DSM 2P Doppler Segmental Sphygmometer, Sonotechnic GmbH) and Doppler ultrasonography (Vivid 7) of the leg vessels. The performed laboratory tests included: a complete blood count with a differential chemistry profile including blood urea nitrogen, creatinine, uric acid, serum protein, CRP, fibrinogen, alanine aminotransferase and aspartate aminotransferase; a lipid profile with total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides; a coagulation profile with PT, APTT and INR; the levels of fasting glucose and glycosylated haemoglobin; and urinalysis. The treatment with the newly developed dressings (called Lenplast BIS) was part of a complex ulcer therapy regime that included education, analgesic and anticoagulant therapy, and compression therapy that were used in an unchanged way by the patient before the study. The total duration of the study was 12 weeks. The treatment was divided into three stages (4 weeks each) preceded by stage zero, in which each patient’s wound was treated with popular, widely available cotton dressings wetted with an isotonic salt solution. This part of the study was treated as the control stage. In the first stage, patients were treated with flax dressings wetted with an isotonic salt solution (Lenplast BIS 1). In the second (Lenplast BIS 2) and third (Lenplast BIS 3) stages, the dressings moistened with oil emulsion and seedcake extract, respectively, were used. After each week, during a consultation, a physician performed an evaluation of the ulcers, measuring the ulcers using sterile dressings with a millimetre scale, and reading a questionnaire filled in by the patients the day before each visit. The physicians also prepared photographic and descriptive documentation. All the dressings were changed every 24 h; the first was applied by qualified hospital personnel. Thereafter, the patients were instructed by a qualified nurse and changed the dressings by themselves. The study was approved by the local bioethics committee. All the patients were provided with written information on the purpose and design of the study.
Measurement of wound dimensions
Wounds were photographed using a fixed focal length lens to ensure the same point of view and scale by the same study team member. To have a correct scale indicator, a commercially available industrial photographic colour standard table was photographed along with the wounds. It was used both to set correct colours of the image and to measure the wound. When the wound was too big to fit on a single image, several individual images were taken around the wound to have a good view for wound surface measurement. Wound measurements were conducted using photographic software by counting pixels within the wound borders. As a reference, the number of pixels inside a 1 cm2 square was used. The 1 cm2 square was photographed for each image. If the wound was too big to fit on a single picture, all fragments on successive pictures were summed.
Results
Biochemical characteristics of wound dressing
Low-molecular phenolic compounds in flax dressings
The phenolic component content was quite similar in Lenplast 1 and 2 (45–50 µg/g), but significantly increased in the case of Lenplast 3 (140 µg/g), which was moistened with a seedcake extract, which provides an additional pool of antioxidants, such as benzoic acid derivatives and more importantly lignan diglucoside (SDG). The detailed results are presented in Supplementary Table 4.
Moreover, flax fibre is a natural source of cannabidiol (CBD). We have measured the concentration of this compound to be at a concentration of 5.03 ±0.28 ng/g.
Fatty acids in flax dressings
Five main fatty acids constitute linseed oil. Palmitic acid (6%), stearic acid (2.5%), oleic acid (19%), linoleic acid (13%) and α-linolenic acid (55%) are the most frequently found fatty acids in flax oil. Fatty acids determined in the three types of flax dressings originate from two sources (Supplementary Table 5): the fibre itself and fibre’s moisturizing oil emulsion. The level of all five fatty acids is significantly increased in Lenplast BIS 2 or slightly increased in Lenplast BIS 2 and 3, respectively, due to the oil emulsion and press cake extract used for moisturizing. The only slight increase of fatty acid contents can be noted in Lenplast BIS 3, which derives from residual oil commonly present in press cake and extracted together with phenolics upon alcohol extraction.
Sterols in flax dressings
The total level of phytosterols in all three tested flax dressing types was almost identical, with β-sitosterol reaching the highest level of about 400 μg/g of dry mass. However, of eight identified derivatives, two discriminate Lenplast BIS types. Cycloartenol is increased by about 41% and 84% in Lenplast BIS 2 and 3, respectively, compared to Lenplast BIS 1. Also 4-methylsterol content is higher in both Lenplast BIS 2 and 3 (64% and 42%, respectively) compared to type 1. Detailed analysis of sterols is presented in Supplementary Table 6.
Compounds in vitro release from dressings and stability of bioactive compounds during storage
The bioactive compounds release from dressings comes as a viable and beneficial substitute in order to improve the healing process of the chronic wound. For example, antiradical properties of phenylpropanoids have demonstrated that they can inhibit lipid oxidation in different systems [1]; thus, phenolics are proposed for the formulation of novel dressing products. Moreover, they are directly associated with the protection of the wound against infection and thus minimizing risks of long-term wound healing.
Stability of bioactive compounds in the integrated dressing
The use of phenolics and terpenophenolics, the main bioactive compounds in our dressings, may be limited due to their instability during storage (light, oxygen). This factor limits the beneficial properties and potential health benefits of these compounds. Controlled delivery systems, such as the integrated dressing, where molecules are associated with lignocellulose polymers of fibres, have shown their potential to protect, control the release, and increase the action of different bioactive compounds. Therefore, in the present study, we have carried out an investigation of bioactive compounds stability and their releases from the integrated dressing into the aqueous environment. Table 1 demonstrates the content of bioactive compounds in the integrated dressing (Lenplast BIS 3) versus a single seedcake extract, which was used as an essential component of this type of the dressing, during storage within 12 months in a standard fridge. It was clearly shown that preparation of the integrated dressing significantly increases the stability of active compounds.
Bioactive compounds in vitro release from the integrated dressing
The compounds in vitro release tests were performed using the chemical protocol described by Gąsiorowski et al. [23]. Table 2 shows the amount of compounds release from the integrated dressing into aqueous solutions after 12 h incubation. The released compounds were detected and measured by UPLC-MS method. The compounds concentration was calculated using standard calibration curves. The compounds content remaining within the lignocellulose polymers of the dressing was calculated from the difference between the total amount of compounds added and the amount of compounds released in the aqueous medium.
Additionally, all integrated dressings released polyhydroxybutyrate, which degrades to D,L-β-hydroxybutyrate in contact with body fluids (data not shown). Similar results were obtained with the flax fabrics incubated for 48 h in cell culture media – all identified phenolic compounds were released to the cell culture media.
The migration potential of bioactive compounds across the semipermeable membrane – an in vitro test
The in vitro release rate may reflect the combined effect of several physical and chemical parameters, including solubility and particle size, and rheological properties of the bioactive compounds. The most popular method uses an open chamber construction, such as the Franz diffusion cell system. The membrane separates the donor compartment containing the test product from the receptor compartment filled with the collecting medium (PBS). Measurement of the release of active compounds from the gel formulation of Lenplast BIS 3 is presented in Figure 1.
Figure 1
Rate of in vitro bioactive compounds release from the extract of integrated dressing (Lenplast BIS 3) in gel form. Lenplast BIS 3 (10 g) was incubated 12 h in buffered saline solution and directly after preparation of gel formulation in carbopol transferred to donor chamber. The cumulative amount (% of total content) was plotted versus time. Data are represented as mean ± SD (n = 6)
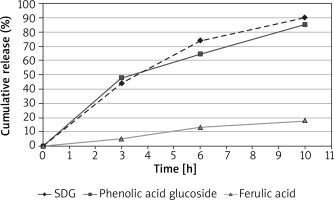
The data have shown the rate of compounds release from the integrated dressing into aqueous solutions after 12 h incubation. The released compounds were detected and measured by UPLC-MS method. The compounds concentration was calculated using standard calibration curves.
The active substances are gradually released (in line progress) from the gel formulation, reaching the maximum at 10th hour. Almost complete releasing concerns phenolic acids glucosides and lignan. The aglycone of phenolics releases on a much smaller scale perhaps due to lower solubility in the water solution. The data suggest the potential of phenolics in cell membrane penetration and being involved in intracellular regulation of oxidative homeostasis and other biological processes.
Antioxidant capacity
The antioxidative capacities of extracts from the dressings were measured using DPPH and TBARS assays and the data are presented in Figure 2. It turned out that moisturizing the flax fabric with oil emulsion or the seedcake extract increases the antioxidative status of the dressing. The increase by 7% in the case of Lenplast BIS 2 compared to Lenplast BIS 1 was statistically significant (p < 0.05) (Figure 2 A). The effect for Lenplast BIS 3 was only slight and not statistically significant.
Figure 2
The antioxidative capacities of extracts from the dressings DPPH and scavenging assay for dressings. Standard- standard cotton gauze (A). TBARS formation measured at 535 nm of oil (cv. Linola) in the presence of extract from dressings (B). Bars represent standard deviations of 6 independent experiments (biological replicates). Asterisk shows statistically significant data (p < 0.05)
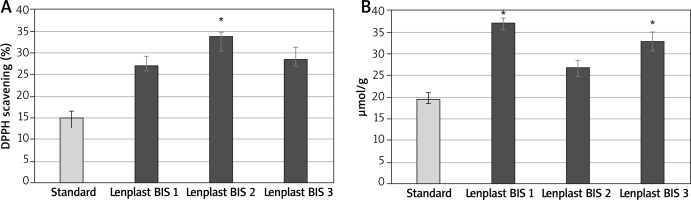
These results were positively verified by the measure of TBARS formation. Those products resulted from the interaction of secondary products of lipid peroxidation and thiobarbituric acid (TBA). Freshly prepared wild-type flax oil (cv. Linola) was used as a substrate in the reaction.
As shown in Figure 2 B, the extracts from dressings moisturized with oil emulsion (Lenplast BIS 2) or the seedcake extract (Lenplast BIS 3) showed better protection against oxidation than the one from the dressing moisturized with the salt solution (Lenplast BIS 1).
The discrepancy between antioxidant content and antioxidative properties measured by the two tests may result from the fact that the majority of the phenolic antioxidants are present in a less active glycosylated form.
The cell density/cell proliferation
Fibroblasts with keratinocytes play a crucial role in the wound healing process progression. Due to the fact that in all types of investigated flax dressings, the presence of substances with potential proproliferative activity – PHB and CBD was confirmed for use in testing the hydrophobic extract in which these substances are present. To determinate the overall influence of the hydrophobic extract from flax dressings (Lenplast BIS 1, 2, 3) on skin fibroblasts, we observed their morphology of cells and they ability to metabolize the harmful substance (MTT test) in the presence of the extract. This type of observation is a commonly known measure of cell proliferation potential. We did not observe any changes in fibroblast cell morphology caused by the flax dressing extract. Basic proliferation potential tests of human fibroblast cells after incubation with extracts from all types of flax dressings revealed a slight positive effect on the cells (Figure 3). The observed influence is statistically significant, yet not strongly related to the type of the dressing.
Figure 3
Proliferation percentage of NHDF cells in in vitro MTT test. Individual bars corresponds to the NHDF cells treated with the methanolic extract from Lenplast BIS 1, Lenplast BIS 2, Lenplast BIS 3. The MTT assay were performed after 72 h of incubation in triplicates.
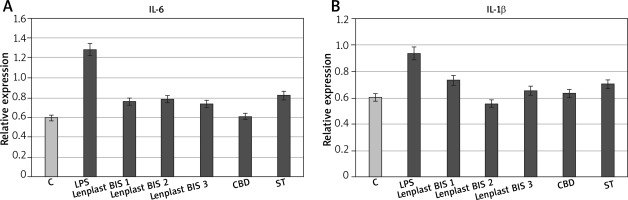
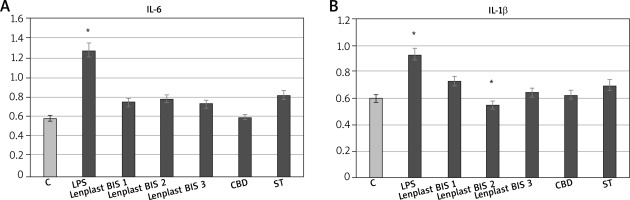
Compounds present in the hydrophobic extract of flax dressings, and in particular, cannabidiol is a well-known anti-inflammatory agent proposed to be responsible for the majority of changes in expression induced by the extract. Its properties decrease mainly expression of proinflammatory genes and increase anti-inflammatory genes, affecting the activation of transcription factors of the pathways associated with protein G. It was demonstrated that gene expression from these pathways has been lowered by cannabinoids of flax in previous microarray studies [18]. Therefore, the expression of crucial genes of inflammation state propagation (IL-6 – one of the most important and most multidirectional cytokines, and IL-1β – this cytokine is an important mediator of the inflammatory response and is involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis) were analysed using the RT real-time PCR method (Figure 4). The results of real-time PCR showed inhibition of proinflammatory gene expression in skin fibroblasts, caused by the flax extract, which is statistically significant.
Figure 4
RT real-time PCRs of genes related to inflammatory state expressed by NHDF cells after treatment with dressing hydrophobic extract. C – untreated cells, LPS – cells with induced inflammation, Lenplast BIS 1, 2, 3 – cells treated with hydrophobic extract from dressings. CBD – control samples treated with pure CBD in concentration (0.1 g/ml); SIT control samples treated with pure β-sitosterol in concentration 1.82 g/ml. Measurements performed in five replicates. Asterisk shows statistically significant data (p < 0.05)
Cell migration
The process of skin cell migration is one of the most important phases of wound healing. It is associated with the induction of proliferation, remodelling of the extracellular matrix, expression of adhesion molecules and growth factors, and morphological changes in cells. Simple migration tests, “wound scratch tests”, made on fibroblasts revealed that in fact an extract from flax dressings affects this process. Results (shown in Figure 5) indicate activation of fibroblast migration, noticeable even after 6 h of incubation (especially in Lenplast BIS 3).
Figure 5
Dressing extract influence on wound closure ”scratch assay” Lenplast BIS 1, Lenplast BIS 2, Lenplast BIS 3 – Fibroblasts treated with extract from 1 dm2 of dressing. C – untreated cells. Measurements of four biological replicates. Asterisk shows statistically significant data (p < 0.05)
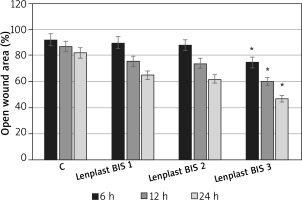
Since in the case of a “wound scratch assay” type of experiment the effect of migration and proliferation cannot be truly distinguished, the wound closure observed may be a sum of these processes, as it is in vivo.
The anti-inflammatory properties of the extract are mainly due to the CBD content (in most cases acting in synergy with β-sitosterol). The role of other extract components (e.g., polyunsaturated fatty acids) is indicated by the literature as possibly responsible for some anti-inflammatory but mostly migration and proliferation promoting action.
Antibacterial property of linen dressings
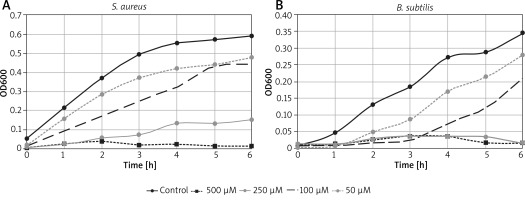
According to previously published data [24], the content of vanillin that is present in 1 dressing (1 dm2 of fabric), 379.09 µM (57.69 µg) for Lenplast BIS 1, and 433.55 µM (68.92 µg) for Lenplast BIS 2 and 492.44 µM (74.95 µg) for Lenplast BIS 3, is sufficient to significantly inhibit S. aureus and B. subtilis growth (Figure 6). For B. subtilis bacteria for all types of the dressing, vanillin concentration is higher than the minimal inhibitory concentration (MIC) of 347 µM. This observation significantly supports antimicrobial activity reported by patients in the examined dressings.
The monomer and polymer of 3-hydroxybutyrate influence the expression of the histone acetyltransferase and histone deacetylase genes
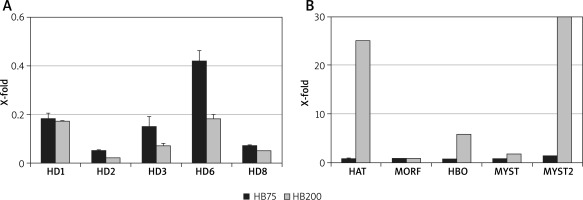
The content of PHB in the flax wound dressing ranges from 46.8 to 51.1 µg/g. In an in vitro experiment, incubation of PHB standard solution with body fluids releases 3-hydroxybutyrate (HB) [25]. According to our experiments, 0.204 µg of HB per mg of PHB and analysis of medium afterwards resulted in PHB disintegration and monomer (HB) appearance. In another in vitro experiment, NHDF cells were incubated with HB in a concentration similar to that resulting from PHB presence in a wound dressing, and expression of genes encoding histone deacetylases and acetylases was analysed (Figure 7). Similarly to the results reported in published data, HB inhibits expression of all five human deacetylase genes in a similar manner. However, the most interesting and novel data came from investigation of five acetylase genes. Two of them, HAT and MYST2, were reasonably activated by HB. The dramatic induction of MYST2 (400-fold) is of special importance since the enzyme modifies histone and non-histone proteins involved in several essential cellular processes, such as gene regulation, DNA damage response, gene dosage compensation and cell viability.
Figure 7
Activity of genes encoding histone deacetylases and acetylases. Relative quantification of human fibroblast histone deacetylases (A) and acetylases (B) gene expression upon treatment with two HB concentrations (75 and 200 μM). The expression level on the non-treated control is always 1. Bars represent standard deviations of 3 independent experiments (biological replicates). Control level – fibroblasts not treated (always 1) were visualized by means of a dashed horizontal line
Novel treatment option with significant evidence for the acceleration of ulcer healing
To evaluate changes in the ulcers yielded by flax fabric treatment, a single parameter with the most objective character was considered, namely the wound size. The sizes of the ulcers measured after each 4 weeks of treatment are presented in Supplementary Table 7. The mean decrease in wound area was most considerable after the first stage (22.7%), and in the following stages the area continued to be reduced relatively to the preceding one (by 21% and 24% in stage 3 and stage 4, respectively). The changes were not statistically significant.
Granulation of the wound started abundantly after the first stage of wound treatment (in 86% of patients) and continued through stages two (in 100% of patients) and three (in 63% of patients). Exudations were observed to be reduced (in 52% of patients in stage 1, in 95% of patients in stage 2 and in 89% of patients in stage 3). Moreover, patients reported diminution of the sense of pain throughout the whole treatment procedure (in 81%, 91% and 95% of patients in stage 1, 2 and 3, respectively).
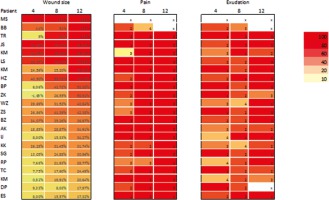
Favourable differences, but without reaching statistical significance, were observed in the level of inflammatory markers (including erythrocyte sedimentation rate, CRP, fibrinogen, platelet count, total leukocytes and neutrophils) between the end of stage 0 and the end of the project (after 12 weeks of treatment). Changes in significant medical parameters observed during 12 weeks of treatment with flax dressings are presented in Supplementary Table 8. A graphical diagram of the progress achieved in the treatment of wounds, ranked in order of severity of the reduction in wound size (from 100% to 17%) is shown in Figure 8.
Figure 8
The progress achieved in the treatment of wounds. Example of wound at successive stages of treatment with Lenplast BIS dressings. The presented colors show the scale of improvement of the most important parameters of wound healing (size of the wound, feeling of pain, exudation from the wound)
Discussion
A chronic wound is often described as one that is physiologically impaired as a result of healing process disruption due to an impaired angiogenesis, cellular proliferation and migration, or other reasons. There is no specific time frame that clearly differentiates an acute from a chronic wound. One suggestion is that 50% reduction of the surface area of the wound over 4 weeks indicates a chronic state and the others point out that a wound that is present for more than 3 months might be treated as chronic [26]. Examples of chronic wounds include non-healing or infected surgical or traumatic wounds, venous ulcers, pressure ulcers, diabetic foot ulcers, and ischemic ulcers. However, the most common chronic wounds are leg ulcers (venous, arterial, mixed), pressure wounds and skin tears. The management of ulcers is complex. Much of the focus in wound management is on the dressing. In general, the dressing does not heal the wound, but appropriate contemporary dressings help to control the micro-environment by controlling the flow of exudate from the wound into the dressing, protect against infection and may also stimulate activity in the healing cascade and speed up the healing process [27].
Topical treatment aims to reduce pain and itching, minimize infection, exudate and bleeding from the wound and to cope with the most troublesome problems of chronic wounds that affect the patient physically and emotionally (e.g. excessive exudation, unpleasant odour) [28].
In recent years, classical treatments such as surgical debridement, disinfection/cleansing of the wounds, compression therapy, larval therapy, in the form of a Bio-FOAM dressing, hydrogel, a variety of wound dressings and novel advanced therapies such as bilayer living cellular construct, topical application of cytokines cocktail secreted by fibroblasts and keratinocytes or the isolated cells themselves, cell therapy containing growth-arrested allogeneic neonatal keratinocytes and fibroblasts, the application of autologous platelet-rich plasma containing fibrin and high concentrations of growth factors, the application of extra corporal shock wave (ESW) therapy, demonstrate a great progress in supporting chronic wounds healing [29]. The ideal dressing should achieve rapid healing at reasonable cost with minimal inconvenience to the patient [30]. Further development of biotechnology resulted in bioactive wound healing dressings that include tissue-engineered products derived from natural or artificial sources [31]. These biomaterials have the advantage of forming part of the natural tissue matrix, are biodegradable, and play an active role in normal wound healing and new tissue formation [32]. In some cases they are supplied with active compounds, such as antimicrobials (antibiotic drugs: gentamycin, ofloxacin, minocycline, bismuth), and may be impregnated with silver or enriched with growth factors (transforming growth factor-β1 – TGF-β1) [33, 34], PDGF [35], human growth hormone (hGH), endothelial growth factor [36], FGF and TGF [37] for delivery to the wound site.
A completely new option is plant biomaterials. Since they contain a broad spectrum of biologically active compounds, they may be used as natural vehicles for delivering their own therapeutic agents to the wound bed and in this way neutralizing free radicals and their products in the wound, handling of fluid, promoting cell proliferation and migration, and serving as a physical wound barrier. They are biodegradable, have suitable tensile strength and elasticity and might be easily used with a simple scheme. These features of biomaterials exactly correspond to flax fibre properties derived from genetically modified flax plants.
At the cellular level, ulcer occurrence is the result of the impairment of normal cell activity on the one hand and an elevated amount of oxidative factors on the other. Reactive free radicals promote pathogenesis and progression of ulceration by impairing numerous cellular mechanisms and causing cell apoptosis. It is already well evidenced that oxidative species have a great impact on chronic wound progression [11]. This was one of the reasons to engineer flax fibres for an increased quantity of naturally synthesized antioxidants and exploit them for wound treatment. Three kinds of compounds were considered to be beneficial for wound healing. The first group constitutes phenylpropanoids and isoprenoids as antioxidative and anti-inflammatory compounds; unsaturated fatty acids as anti-inflammatory and cell membrane regeneration metabolites belong to the second group; and the third group includes 3-hydroxybutyrate as the activator of genes that control histone and non-histone modification and thus promote cell proliferation. The first group of compounds is represented by phenolic acids, flavonoids, lignans and benzoic derivatives with the primary function as free radical scavengers. The function of these compounds is quite well known. However, recent findings indicated the function of phenolics also in protection of the wound against microbial infection. It was reported that the extract from the flax seedcake killed several hospital strains of bacteria that complicate wound healing [13]. This positive effect was shown for Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and also for fungi such as Candida albicans. Sterols and terpenophenol – cannabidiol (CBD), and also the constituents of the first group of compounds in flax fibre, participate greatly in anti-inflammatory action. For example, it has been found very recently that CBD from flax fibre activated an anti-inflammatory gene and suppressed pro-inflammatory gene expression in human fibroblasts and keratinocytes [19]. CBD activity results in inhibition of expression of many cytokines, including IL-1, IL-6, MCP-1 and TNF-α. It has also been shown to directly diminish the activity of inflammation induced transcription factors, such as NFκB [38]. The presence of CBD is also connected to COX 2 and NOS expression inhibition, which causes lowering of ROS production and reduction of inflammation propagation [39, 40]. Other hydrophobic components, in particular plant sterols present in flax fibre also positively affect cell migration and proliferation [19]. β-sitosterol was shown to have a pro-angiogenic and migration-promoting impact on human endothelial cells [41] and keratinocyte cell line proliferation [42].
The second group of flax wound dressing components consists of unsaturated fatty acids, with the highest representation of linoleic acid (LA) and α-linolenic acid (ALA). ALA can reduce the production of arachidonic acids and other pro-inflammatory eicosanoids. The linseed fatty acids affect expression of cytokine genes, such as IL-4 and IL-8 genes [43]. The impact of the form of administration of oil on the organism functioning is also an interesting issue. For example, linseed oil in microemulsion or nanoemulsion appears to be useful as a carrier of therapeutic agents for transdermal delivery [43]. Omega-3 and omega-6 fatty acids have been reported previously as having a beneficial influence on wound healing in animal models [44].
The third group of key components of the wound dressing is 3-hydroxybutyrate polymer (PHB). It has been shown that polyhydroxybutyrate in contact with body fluid degrades to release monomers, which activates cell proliferation. Its known function as a deacetylase gene expression inhibitor is now strengthened by a novel function as a specific and extremely strong activator of acetylase gene expression. The target genes for highly activated MYST acetylase are those coding for the DNA damage response, gene dosage compensation and cell viability [45].
It is important to point out that suitable wound dressings include bioactive compounds that are released to the wound and penetrate cell membranes. It was demonstrated that generated flax fabric released a detectable amount of phenolic compounds to aqueous media, and thus, most probably to the moist wound environment. Further, the compounds passes through the semipermeable membrane with certain kinetic, which suggests that they penetrate the cell membrane.
Thus, accumulated biochemical data, strengthened by flax fibre’s high water absorption (ca. 20% w/w) property, strongly suggest the usefulness of novel dressings for wound healing. The function of the three groups of biochemical compounds corresponds quite well to the recognized stages of wound healing. However, we cannot exclude the influence of other, not yet recognized factors.
In this study several beneficial effects were detected during human treatment. There were two kinds of effects observed almost from the beginning of the pre-clinical trial. The first was reduction of chronic pain associated with ulceration, reported by treated patients. The second was the reduction of mass of fibrin in the wound bed, documented by photos.
Then, after 4 weeks (after stage 1) of treatment, progressive drying of wounds was observed, connected with the optimized exudate control (removal of excess exudates). During stage 2, and especially at the end of this stage, significant quantities of granulation tissue in ulcerations were detected. Due to the arrangement of the dressing’s fibres and its adhesion to the wound bed, the newly formed granulation tissue never hindered the healing process. The most important was the final result – proven reduction of wound size, due to epithelialization – formation of new epidermis. Flax dressings, although adhesive to the wound, were pain free and easily changed by patients.
In summary, from pilot clinical trials we have learned that the induction of wound healing by Lenplast was independent of the patient gender, age, duration of the ulcer, size of the ulcer, previous type of therapy, concomitant diseases, and the underlying pathophysiology (Supplementary Table 2). Encouraged by these results, bioactive flax dressings can be suggested as valuable support of routine, daily ulcer treatment in addition to classical ulcer care. However, it should be also pointed out that this conclusion is based on the data from single small randomized controlled trials.
Taken together the flax dressing appears to be the optimal wound coverage. It supports necrotic debris removing, absorbs exudate, is useful for extremely exudative and infected superficial wounds, protects surrounding healthy and fragile skin, is permeable to oxygen, beneficial for bandaging a challenging anatomical area and containing various other topical agents that have been used for wound management including antioxidants, inducers of cell proliferation and migration, compounds affecting cell membranes regeneration and pain relief.
Conclusions
Collected data indicate that applied flax dressings affect multiple stages of the wound healing process, what makes them unique among currently available products. The effect of wound healing by flax dressings results from the concert action of different compounds (phenolics, sterols, lignans, CBD, PHB), however, the role of PHB should be emphasized. Their effects on wound healing were proven both in in vitro studies (elimination of free radicals, antibacterial effect, stimulation of proliferation of fibroblasts, endothelium and keratinocytes) and in a pre-clinical pilot trial (elimination of excess of exudates, normalization of gas exchange, providing proper humidity, stimulation of proliferation of fibroblasts, endothelium and keratinocytes from wound margins). A positive effect of applying the flax-dressings was observed in all patients attending the study, even in those, in which other therapies were ineffective. Moreover, the ease of use and pain reduction are additional positive aspects of the examined dressings, which surely encourages the patients to use them. Due to the limited number of patients in the study it was impossible to perform additional tests (different times and order of stages, non-transgenic flax control). Based on our studies on the GM-flax fibre composition and the influence of the GM-flax fabric on fibroblast proliferation, we designed the study to employ only the most effective dressing, that is those passed on GM-flax. Further optimisation of the wound dressings based on GM-flax fibre is the next obvious step.