Introduction
Wound cleansing is an inseparable element of wound healing therapy. The term “debridement” is used as an international medical term. It stands for removal of necrotic material, scabs, devitalized tissues, dried serous fluid, infected tissues, biofilm, stratified epidermis, pus, hematomas, foreign bodies, bone fragments and other impurities whose presence delays wound healing.
According to the European Wound Management Association’s guidelines, properly performed debridement leads to improvement of microcirculation in the wound, reduction in inflammation and lowering of the level of metalloproteinases, stimulation of wound edges and epidermis, reduction in unpleasant odour and reduction in the risk of infection and improvement of the patient’s quality of life [1].
There are many debridement techniques approved by the European Wound Management Association. According to the new TIMERS guidelines, the selection of the most appropriate method depends on many factors, namely tissue type, presence of biofilm, depth and location of the wound, underlying cause (venous, arterial), skills of the person performing the debridement and the preferences of the patient him/herself [1, 2].
Some methods of tissue cleansing can only be applied in specialized hospital wards with ultrasonic wound cleaning devices. Consideration should be given to pain in the area of ulceration, the possibility of effective anaesthesia, the cost of the method, the environmental conditions in which the procedure is performed, having the appropriate authorization to perform the cleansing procedure, and guidelines on recommended debridement techniques.
The wound cleaning technique include (Figure 1):
Figure 1
The wound cleaning techniques used in outpatients include: 1–2 – monofilament cloths, 3–4 – sterile sponges, 5–6 – dressings made of poly-absorbent fibre, 7–9 – alginate dressings, 10 – collagenase ointment

Mechanical wound cleansing
Mechanical debridement is a debridement method that uses physical strength to remove necrotic tissue. These techniques are most often used for initial tissue cleansing preceding other debridement methods. The biggest problem with mechanical debridement techniques is that they are nonselective and both viable and non-viable tissues can be removed [3]. They also require premedication as they may lead to episodic pain [4]. Special caution should be taken when treating the patient who takes anticoagulants as it can be a contraindication for sharp debridement (when INR is > 2.5). Such a patient should be observed longer after the debridement procedure is performed to manage possible bleeding [2].
Debridement with surgical tools and wet-to-dry dressings are the most commonly used form of mechanical debridement. Therapeutic irrigation (delivered by pulsed lavage or the agitation of water during whirlpool therapy) and ultrasound therapy are more sophisticated forms of mechanical debridement [5].
Wet-to-dry technique
The “wet-to-dry” method involves applying gauze dressings impregnated with antiseptics or lavaseptics directly onto the wound, followed by covering the wet dressings with dry ones [6]. This is an archaic method, although it seems that it is still commonly used in both the United States and Poland. Despite a number of disadvantages such as low effectiveness, increasing risk of infection in the wound, pain associated with dressing change, necessity of frequent dressing change; the method is used due to the speed of application, no need for special qualifications and the wide availability of sterile gauze in treatment rooms [6].
Cleansing sponges
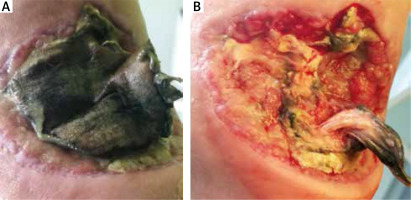
Modification of the wet-to-dry method is sterile sponges, which are soaked with antiseptic fluid. According to the manufacturer’s recommendations, the sponge with coarse texture and rough surface is used to wash the wound from necrotic tissues, while the fine texture sponge should be placed in the wound soaked with antiseptic fluid and then covered with a dry sterile dressing (Figure 2) [5]. This antiseptic fluid, due to a surfactant’s properties, enhances the removal of the non-viable tissue. This is a result of lowering the surface tension (or interfacial tension) between two liquids, between a gas and a liquid, or between a liquid and a solid [7]. It aids in aggregating and consequently removing the necrotic tissue.
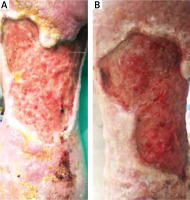
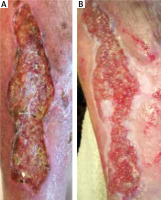
Figure 2
The effect of debridement with a sterile sponge twice a week after 2 weeks in B. 55-year-old male patient with a 2 year old venous ulcer with abundant necrosis on the left leg. The ulcer developed after erysipelas. Doppler ultrasound confirmed the insufficiency of the saphenous vein valves
Blood flow is stimulated around the wound bed which encourages the body’s natural reactions to healing contaminated or infected wounds. This consequently leads to the promotion of granulation.
Cleansing with monofilaments
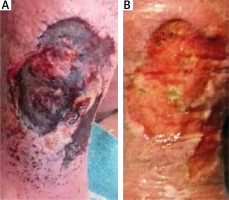
Another alternative to gauze is cleansing with special disposable cloths. There is a product that consists of a soft, dense nap of monofilament, 100% polyester fibres knitted to the reverse side and secured with polyacrylate [8]. It is for single use only. Cloths are a rapid, highly effective, safe and easy method of debridement for superficial wounds containing loose slough and debris (Figure 3). This includes leg ulcers, pressure ulcers, diabetic foot ulcers, and post-operative wounds healing by secondary intention. It is also very effective in the removal of hyperkeratosis from the skin [9]. Debris and exudate are actively loosened from the wound by the fibre. Skin flakes and keratoses are also removed from the surrounding skin [10]. The fact that this procedure is significantly less painful than conventional methods is worth noting.
Figure 3
The effect of one-time debridement with a monofilament cloth. 82-year-old female patient suffering from chronic venous insufficiency known for more than 20 years. At the time presenting a 2 year old exuding ulcer on the right leg with maceration of the surrounding skin
A monofilament debridement pad, according to the NICE (National Institute for Health and Care Excellence) Medical Technology Guidance, remained cost saving in most analyses and savings ranged from £77 to £222 per patient compared with hydrogel, from £97 to £347 compared with saline and gauze, and from £180 to £484 compared with larvae depending on the assumptions included in the analysis and whether debridement took place in a home or clinic setting [8].
Cleansing with surgical instruments
The most expensive example of the mechanical cleansing method is sharp debridement performed under general anaesthesia in the operating theatre. Nevertheless, surgical debridement should be considered whenever the goal is to quickly remove large amounts of necrotic tissue [11].
In the operating theatre plenty of different methods could be performed, from sharp to ultrasound debridement. Sterile conditions have several advantages, including the possibility of quick and very accurate excision of all necrotic tissues without worrying about the patient’s pain. It also enables physicians to simultaneously combine different methods like wound cleansing and skin grafting or negative pressure wound therapy.
An effective and simple debridement method is to remove impurities using sterile surgical instruments: Volkmann bone curette spoon, a scalpel, or tweezers. This is a quick way to get rid of necrotic tissue from the wound. Mechanical cleaning with a spoon should be an inseparable element each time the dressing is changed in the conditions of the hospital ward and the treatment room (Figures 4, 5). Careful use of a scalpel makes the procedure quick and painless, and the effect we can achieve with this method often replaces long and arduous dissolution of the necrosis with the help of dressings (Figure 6). Volkmann spoon is an indispensable tool in the clinics where wounds are processed. It allows us to remove the biofilm and yellow fibrin from the wound [12]. The patient should receive 2% lignocaine gel on the wound, embedded in an occlusive dressing for at least 30 min, prior to using the Volkmann spoon.
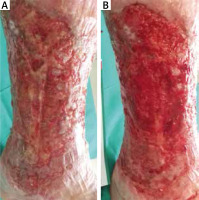
Figure 4
Surgical debridement method using Volkmann spoon. The area subjected to this form of debridement should be anaesthetized, preferably using occlusive lignocaine dressing
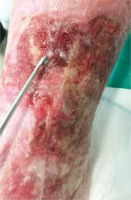
Figure 5
The effect of surgical debridement using Volkmann spoon, a week after the procedure. 32-year-old male patient presented with a two year old leg ulcer and severe lipodermatosclerosis on his left leg. The ulcer had uneven raised edges. The patient has been suffering from chronic venous insufficiency and deep vein thrombosis for 5 years
Ultrasound wound cleansing
Ultrasound debridement is a promising technology that stimulates wound healing by decreasing exudate and slough, decreasing patient’s pain and dispersing the biofilm [13]. Most commonly used low-frequency ultrasound used for cleansing ranges between 20 and 60 kHz and has longer wavelengths and greater amplitude for a given input energy, which results in greater movement of molecules within tissues [14]. Low-frequency ultrasound debridement enhances removing devitalized tissues through microstreaming and cavitational effects [13]. Ultrasound wound cleansing is more selective than previously described methods.
It emulsifies non-viable tissues with microsize gas bubbles and at the same time promotes healing by upregulating cellular activity, promoting protein synthesis and fibrinolysis, as well as disrupting the biofilm [15, 16].
A randomized double-blind controlled trial has compared low-frequency low-intensity ultrasonic debridement to a sham treatment (saline mist without ultrasound) in patients with recalcitrant diabetes-related foot ulcers. Ennis et al. found that after 12 weeks of treatment 40.7% of patients who underwent LFUD had healed compared to only 14.3% in the sham treatment group [17].
Hydrosurgery
Hydrosurgery uses a high-pressure jet of sterile saline (0.9% sodium chloride) to debride wounds and, through a localized vacuum effect on the surrounding tissue, promotes cutting and aspiration of the devitalized tissue [18]. It allows to cut, remove soft tissue and reduce the bacterial load in the wound. Hydrosurgery is a highly advanced technique that enables the operator to adjust location and depth of the cut. It is a fast and easy method for cleansing both acute and chronic wounds. Moreover, it has been proven that it allows removal of necrotic tissue and drainage of infected tissue by reducing the bacterial load and restoring a vital wound bed [19].
A prospective controlled study comparing hydrosurgery with conventional surgical debridement carried out by Caputo et al. on 41 patients with leg ulcers has shown that time needed for debridement was 10.8 min compared with 17.7 min and thus significantly shorter for the hydrosurgery group. On the other hand, the clinical efficacy did not differ significantly between the groups. The median wound healing time was 71 days for hydrosurgery and 74 days for conventional surgical debridement [20]. It has been stated that the average VAS score was lower in patients treated with hydrosurgery.
Negative pressure wound therapy
Negative pressure wound therapy, also known as vacuum-assisted closure (VAC), is a type of therapy that decreases air pressure on the wound below the atmospheric pressure. This can help the wound heal faster. By removing the pressure over the area of the wound, it can gently pull fluid from the wound as well as reduce swelling, and may help clean the wound and remove bacteria (Figure 7) [21]. This therapy is particularly advised if there is traumatic tissue loss, if primary wound closure is not possible or if the wound has to be left open or reopened because of an infection [22].
Figure 7
At the first visit (A), after debridement with Volkmann spoon (B) and the effect after 3 months’ treatment with negative pressure system (C). 69-year-old female patient with deep, multiple venous ulcers filled with biofilm on the left leg. Ulcers developed as a complication after the treatment of erysipelas 2 months before
A wound vacuum system has several parts. A foam or gauze dressing is put directly on the wound. An adhesive film covers and seals the dressing and wound. A drainage tube leads from under the adhesive film and connects to a portable vacuum pump. This pump removes air pressure over the wound. It can do it all the time or in cycles. There are also sets that simultaneously enable to rinse the wound with lavaseptic [23]. The dressing is changed every 24 to 72 h. The biggest disadvantage of this method is that during the therapy, the patient needs to carry the portable pump everywhere he or she goes.
Vacuum therapy accelerates the wound healing process due to numerous mechanisms, including the promotion of cell proliferation through mechanical stretching of cells, stimulation of growth of granulation tissue in the wound, increase in blood flow within the wound, removal of wound healing inhibitors found in exudate fluid [24, 25]. Moreover it leads to reduction in oedema, and reduction in bacterial colonization of the wound and prevention of cross-infection through the use of a closed system and keeping the edges of the wound closer together [26].
This method is not only used to cleanse shin wounds, but also wounds found on the chest or abdominal area. It is widely used in patients with complicated postoperative wounds in the abdominal cavity. A retrospective study by Seternes et al. on open abdomen treatment (OAT) has shown that among 118 patients with OAT, primary fascial closure was achieved in 76 (84%) patients surviving the OA treatment.
There are also more publications that demonstrate the efficacy of negative pressure wound therapy in the head and neck. Strub demonstrated that it reduces wound infections, shortens hospital stays, and simplifies wound reconstruction strategies [27]. He also implies that VAC should be included in the wound management strategies of otolaryngologists and facial plastic surgeons. Dhir et al. published a cohort study in which among 19 patients with a variety of complicated head and neck wounds, 84% of patients healed completely after VAC therapy [28].
This directly implies that VAC is a very efficient and promising therapy, which might soon have new indications.
Maggot therapy
Maggot therapy is a type of biotherapy that involves placement of disinfected fly larvae (Lucilia sericata) into a non-healing wound. The base of their action is eating out the necrotic tissue. This is the most selective method known since maggots only eat away devitalised tissues.
There are numerous studies suggesting that maggot therapy is more effective and efficient in debriding chronic venous ulcers, pressure ulcers, and diabetic ulcers. Maggot therapy is also associated with a more rapid decrease in wound size and an increase in granulation tissue, making the wounds ready for surgical closure.
In his study, Sherman evaluated a cohort of 103 inpatients with 145 pressure ulcers [29]. It has been shown than within 3 weeks, maggot-treated wounds contained one-third of the necrotic tissue and twice the granulation tissue, compared to non-maggot-treated wounds.
In another study Sherman has shown that after 5 weeks of therapy, conventionally treated wounds were still covered with necrotic tissue over 33% of their surface, whereas after only 4 weeks of therapy maggot-treated wounds were completely debrided [30]. Moreover, more patients actually achieved a complete wound closure within the 8-week study period (14% with maggot therapy vs. 0% with conventional).
A study on 343 patients all over the UK by Thomas and McCubbin showed that the average daily debridement rate for maggots is 21% (range: 5.6–38%) [31].
Initially, it was thought that debridement with maggots is purely mechanical. Nowadays it has been proven that maggot’s excretions also contain proteolytic enzymes, which enables them to have inhibitory effect on both Gram-positive and negative bacteria including MRSA Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa [32]. Studies performed by Brown et al. and Harris et al. showed that maggot-derived enzymes can reduce biofilms in leg ulcer patients [33–35]. The ammonia excreted by maggots is believed to alter the pH of the wound, which inhibits bacterial growth [36]. Maggot therapy is beneficial for debridement as it reduces biofilm formation and aids wound healing by regulating MMPs and infection [37]. This therapy is still considered controversial by many clinicians, however it is a method widely accepted by prestigious wound management associations.
Nowadays, we can choose from maggots in two forms – classical, free form or closed in a special dressing [38]. Yet a study performed by Blake has shown no significant difference in debriding efficiency between those two types. It is crucial to remember that use of occlusive dressings is forbidden since it prevents oxygen exchange between atmosphere and the wound. For the same reason, use of compressive or negative pressure wound therapy is not advisable.
Among the disadvantages of the maggot therapy, it is very costly and it requires a proper connection between hospital and laboratory harvesting maggots. This is detrimental for proper treatment as maggot’s vitality is affected by inappropriate transport. It is also important to strictly follow the producer’s manual on when to change the dressing, since after the expiry date maggots can transform into insects.
The use of specialized dressings
There are numerous cleansing dressings on the market, which are most effective when used as a second step after mechanical debridement. They are worth considering because of balancing adhesion, atraumatic removal and low bioadhesion. They also provide optimal moist wound healing environment all the way across the surface of the healing wound.
Cleansing-absorbing dressing
It is a dressing whose mechanism of action is based on rinsing the wound with an antimicrobial substance – polyhexamethylene biguanide hydrochloride, and the absorption of dead cellular elements and excess wound exudate, as well as bacteria and extracellular matrix building bacterial biofilm [39, 40]. The dressing is built in such a way that throughout the entire application, i.e. 3 days, it maintains adequate wound pH, humidity, and reduce inflammation by reducing the concentration of metalloproteinases in the wound environment [41]. The dressing eliminates all known local obstacles in the healing process of chronic wounds: restores the biochemical balance in the wound bed, allows removal of the necrotic load, ensures proper moist environment within the wound, destroys the biofilm and reduces the pH value to the physiological level [42]. The use of the cleansing-absorbing dressing for a contaminated chronic wound allows lowering the level of active metalloproteins from the extracellular matrix in the wound bed, removing the residual necrotic layers, absorbing excessive amount of exudate and bringing proper therapeutic moisture into the wound. Furthermore, the dressing absorbs protein content, including all biofilm-building organisms, and lowers the pH to the physiological value [43]. Thanks to the PHMB content, the dressing is an excellent alternative for patients with intolerance to silver [44]. The product is perfect to use with compression therapy despite the large amount of fluid embedded in it. The dressing is available in two types: for deep wounds with or without pockets (a rinsing-absorbent layer is present on both sides of the dressing) and for superficial wounds (the outer layer is made of waterproof material, which allows the dressing to be used as primary and secondary one at once). The figure shows the effect of the dressing after one application on a difficult-healing wound infected with Pseudomonas aeruginosa (Figure 8). The disadvantage is the small size of the dressing and therefore the need to combine several dressings on large wounds.
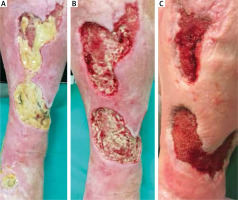
Figure 8
Pictures show the effect of one-time application of cleansing-absorbing dressing. 84-year-old patient with an encircling ulcer of the right leg with abundant biofilm. The ulcer developed 5 years ago, before it was treated unsuccessfully with gauze debridement. The patient has suffered from chronic venous insufficiency and deep venous thrombosis for 25 years
Groenewald in his single blind randomized trial compared wound cleansing times achieved with dextranomer dressing with standard therapy. It has been shown that bacteria and cellular debris present in the wound are taken up by capillary action and become trapped in the spaces between the beads. When the dressing is changed, this debris will be washed away. The beads, which have a high suction pressure (up to 200 mm Hg), have been claimed to reduce local tissue oedema and control odour formation [45].
The mean cleansing time for the dextranomer-treated ulcers was 6 days, compared with 15 for the control group, while the average healing time of the treated ulcers was 4.4 weeks compared with 5.3 weeks for the control groups.
Dressings made of poly-absorbent fibre (Figures 1, 6)
A novelty on the Polish market is a product designed for cleaning wounds in the form of a dressing made of poly-absorbent fibres. The mechanism of knitting the dressing is based on the mechanical penetration of dressing fibre into the biofilm layer covering the ulcer and its destruction when removing it from the wound [46]. The manufacturer recommends initially changing the dressing every 24 h for a better mechanical cleaning effect on the wound, then leaving the dressing for up to 7 days. The effects of the dressing are visible in Figure 9; interestingly, between the changes of dressings no cleansing of the wound with a bone spoon was used. The dressing itself consists of a thin layer of fibres covered on the inside with a patented lipid-hydrocolloid layer that stimulates healing by ensuring an appropriate level of moisture and oiling the wound, as well as providing atraumatic dressing change [47]. The disadvantage of the dressing is its low absorbency. In case of wounds with high and medium exudate, it is necessary to apply a secondary dressing because a thin layer of poly-absorbent fibres has no absorbing properties. The dressing is available in a silver-containing form and without silver, then it is possible to use antibacterial substances such as iodopovidone gel, poly(hexamethyl biguanide) gel or manuka honey [48].
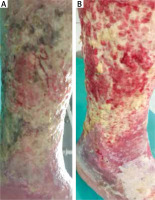
Figure 9
The cleansing effects of poly-absorbent fibres after one application for 3 days (B) and the effect after a week (C). 67-year-old female patient with 9 × 10 cm leg ulcer with uneven edges on the left leg for 5 years. The patient was treated with negative pressure therapy a year ago, yet developed a severe allergic reaction with serous blister formation, followed by their bursting. In 1978 in the same place communication trauma covered with a skin graft
Technology Lipido-Colloid (TLC) Nano Oligosaccharide Factor (NOSF) polyabsorbent fibers inhibits excess metalloproteinases and promotes angiogenesis. The poly-absorbent fibres bind, trap and retain exudate, slough and debris [49].
This dressing has shown better autolytic properties than the control group in the management of venous leg ulcers at the sloughy stage. In a randomized control trial it has been proven to deslough 50% more surface than hydrofiber dressing in a randomised controlled trial [50].
An open-label case study on 13 patients has shown that after 2 weeks treatment with a poly-absorbent fibre pad containing a protease inhibitor (TLC-NOSF Healing Matrix) was associated with rapid improvements that are consistent with wound healing. The mean percentage of healthy granulation tissue in the wound increased from 50% to 60%. The mean wound size reduced from 25.11 cm3 at baseline to 2.60 cm3 after 2 weeks (89.6% reduction). In 90% of the wounds less slough and necrosis was observed, less marked increase in exudate (67% of wounds). Furthermore, 77% of patients rated comfort during wearing as "good" and 85% as excellent during removal of the dressing.
Gethin et al. carried out a multicentre prospective randomized and controlled study on manuka honey compared with hydrogel, performed on 108 patients with venous leg ulcers. A significant reduction of the wound size after 4 weeks (34% vs. 13%, p = 0.001) was observed in a group treated with Manuka honey dressings [51].
Alginate dressings
This is a group of dressings offered by almost every company specializing in the production of dressings. At the same time, they are the oldest cleansing dressings on the market. Alginates are biopolymers of natural origin obtained from marine algae [52]. As natural polymers, they are non-toxic and safe to use. They absorb the fluid from the wound and at the same time form gel and provide a physiological, moisture environment for the wound [53]. When a water-insoluble calcium alginate fibre is placed in contact with wound exudates, the calcium ions exchange with sodium ions in the body fluid and calcium ions are released, which can act as a haemostatic agent [54].
Alginates are now manufactured as wound dressings, such as hydrogels, films, wafers, foams, nanofibers, and in topical formulations [55]. Worth noting, there is a hydrogel containing both alginates and carboxymethylcellulose. It is primarily indicated for the treatment of necrotic leg ulcers, pressure sores and uninfected wounds within diabetic foot. It can also be used for 1st and 2nd degree burns. The gel can be used throughout the treatment period to ensure a moist healing environment for most types of wounds.
Alginates also promote rapid re-epithelialization and granulation tissue formation [55]. Very good effects are obtained by combining amorphous gels and alginate dressings as shown on Figure 10. It is worth noting that sometimes the dressing can smell like natural fresh algae, what can be unpleasant for the patient. There are different types of dressings, ones that dissolve completely and others that do not dissolve completely and leave flocs. Those flocs do not have to be removed completely, since, as most natural materials, they will decay themselves.
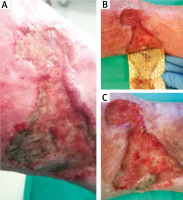
Figure 10
The effect obtained by combined application of amorphous gels and alginate dressings after 1 month. 69-year-old female patient with an exuding leg ulcer that developed a year before. The ulcer was deep and irregularly shaped with medium necrotic tissue abundance
It is also distinctive that it is possible that discolouration of tissues to green occurs due to single settlements (Figures 7–9).
Hydrofiber dressings
Hydrofiber dressings based on carboxymethylcellulose (CMC) can be used particularly on exuding wounds with hydrogels when the wounds are dry. The hydrofiber dressings are recognized for their therapeutic benefit in the healing process of chronic wounds, due to their autolytic properties [56]. Those innovative dressings absorb wound fluid and create a soft gel, maintaining a moist wound environment [57]. It locks in exudate through vertically wicking, reducing humidity in the wound and eventually minimalising the risk of maceration. It substantially minimizes pain during dressing change and while in place [48]. Hydrofiber dressings have been designed to allow optimal fluid transport between the dressings to aid with effective exudate management. A study by Parsons et al. has proven that hydrofiber dressing are suitable for effective exudate management, in terms of fluid handling capacity, fluid retention and low lateral fluid spread across the dressing surface [58].
The high level of absorbency and marked gelling capacity of the hydrofiber dressing offer autolytic properties conducive to local debridement which are atraumatic to the wound and therefore painless for the patient.
Enzymatic products
Enzymatic debridement are based on removal of necrotic tissue through digesting and dissolving the devitalized tissue in the wound. It is a topical treatment that uses natural proteolytic enzymes or proteinases. Proteinase activity is highly useful, since apart from debridement itself it also enhances cell migration that is fundamental for epithelialization. Some enzymes are selective towards non-viable tissue, yet some are nonselective. Enzymatic debridement could be implemented when there are contraindications for mechanical debridement. Enzymatic treatment should not be used when advanced necrosis is observed and the wound is dry as it needs moisture to act. Several enzymatic debridement agents have been developed, such as trypsin, streptokinase–streptodornase combination and subtilisin [59].
Papain is a nonspecific cysteine protease derived from the fruit Carica papaya and capable of breaking down a variety of necrotic tissue substrates. The role of urea is to facilitate the proteolytic action of papain by altering the structure of proteins [60]. Papain is nonselective, targeting for degradation of any protein containing cysteine residues (which are present in most proteins, including growth factors). Papain-urea preparations have been in clinical use for decades – particularly for pressure ulcers – and available literature indicates that these debriding systems are effective when used properly.
Collagenase ointment is another example of enzymatic debridement that is theoretically selective as it breaks down solely collagen that is a major component of nonviable tissue in the wound [61]. It is derived from bacteria Clostridium histolyticum [62]. Collagenase is supposed to be safe in infected wounds, yet it is most effective in physiologic pH. It removes detritus without harming the viable tissue [63]. As a result it enhances granulation and afterwards epithelialization of the ulcer site.
Collagenase can shorten an excessive inflammatory period by down-regulating inflammatory cytokines [64]. It can also promote healing by enhancing cell migration, proliferation and angiogenesis.
A study performed by König et al. on a group of 42 patients with chronic venous ulcers has shown that during the first 14 days the slough within the groups was reduced by almost 19% for wound pad with Ringer’s solution and by 9% for ointment with collagenase, followed by an increase of 26% and 10% respectively in granulation tissue. Although the wound pad with Ringer solution appeared to be more efficient in a few cases, the general efficacy of the two products appeared to be almost the same as no statistically significant superiority of either method as noted [65].
Another study by Waycaster and Milne concentrated on the cost-effectiveness ratio that also derived for hydrogel dressing and collagenase ointment, based on the expected total costs per patient and the clinical benefit conferred, based on the number of epithelialized days occurring across a 1-year time. Patients treated with a hydrogel dressing incurred total treatment costs that were 2.7-times higher than those treated with collagenase. The clinical benefit of collagenase was 1.5-times greater when compared to the hydrogel [66].
Autolytic debridement
Autolytic debridement (Figure 10) involves the use of moisture-donating or moisture-retentive dressings such as hydrogels, hydrocolloids or transparent films, which are placed over the wound and allow the endogenous enzymes within the wound fluid to digest and liquefy necrotic tissue [63]. The dressing is easy to apply and is typically left in place for 2–3 days. After it is removed, the wound should be irrigated with normal saline to remove liquefied debris.
Autolytic debridement is indicated for wounds with necrotic tissue to rehydrate and soften the hard eschar depending on the slough abundance – hydrogels for moderate or no exudate, while absorptive hydrofibers for exudative wounds [67].
Unfortunately this technique is slower and therefore can require multiple dressing applications and irrigations for several weeks or longer. It can also be less effective in older people, especially with chronic wounds connected with the compromised immune system. Autolytic debridement is also not appropriate for infected wounds or very deep cavity wounds that require packing [68].
Amorphous gels and hydrocolloids are intended to clean the wound and provide a moist environment that is favourable for healing [69]. The dressing protects the wound from friction damage, as it provides extra fluid under the dressing. It is also almost painless to change those dressings. For moist environment stimulates recruitment of leukocytes and consequently release of natural painkillers, it provides pain relief. Modifying the pH of the exudate favourably influences the action of sodium and calcium channels that are involved in pain response [70]. Ringer’s solution is one example of those dressings, which has a dilution effect and leads to change in pH that consequently decreases the inflammatory response and reduces negative effect of pro-inflammatory components, such as metalloproteases [71].
A clinical study by Heffernan on 96 patients compared a new hydrocolloid dressing with a non-adherent dressing in the management of lacerations, abrasions and minor operations. While time to heal was similar for both groups, the patients using a hydrocolloid dressing experienced less pain, required less analgesia and were able to carry out their normal daily activities including bathing or showering without affecting the dressing or the wound [72].
A study by Schmidt et al. conclude that, in addition to providing a moist wound-healing environment, certain hydrocolloids might contribute to the establishment and maintenance of the reducing environment necessary for energy production and hence cell division [73]. The release of hydrogen peroxide into the wound environment could conceivably contribute both to the inflammation phase of wound healing and to fibroblast proliferation and hence the granulation phase.
Another study comparing 2 different hydrocolloid dressings has shown that both products performed equally well in a range of wound situations [74]. These materials have the advantages of occlusion, reduced dressing change frequency and absorption.
Conclusions
The increasing incidence and poor socioeconomic outcome of treatment of chronic and difficult-to-heal wounds inspired researchers to work on more efficient and cost-effective ways. Nowadays we have numerous different treatment methods, including new debridement techniques that can be easily performed by patients or their guardians. According to TIMERS strategy, debridement is the first step that is critical for proper wound healing. It has been proven that adequate cleansing can influence the rate of wound closure. The choice of the method has to be based on exudate abundance, infection presence, amount of non-viable tissue and degree of moisture of the ulcer, as well as skills of the physician and other caregivers.
No matter whether immediate removal of non-viable tissue is necessary, surgical or sharp debridement is recommended. On the other hand those methods are not a long-term option, and should be performed by an experienced physician. Combining two or more methods often brings better clinical outcomes. For instance, it is advisable to treat wound with enzymatic cleansing for a week before performing surgical debridement.
Once a wound needs two sequential episodes of sharp debridement, maintenance debridement with collagenase should be employed. This can be augmented by both using autolytic debridement in the form of secondary dressings such as foams; and/or mechanical debridement strategies as well.
First and foremost, the patient’s pain and availability should be taken into account when deciding on the debridement method.