Introduction
In recent years, following a healthy lifestyle has become extremely popular. An important part of such a lifestyle is a balanced diet, which is essential for protecting the body from diseases and maintaining well-being. The important role played by microelements in the proper functioning of the human body has been emphasized in many studies.
The second most abundant trace element, after iron, is zinc. It plays an important role in the body by participating in various processes, both at the cellular and systemic level, contributing to cell proliferation, differentiation and apoptosis, DNA and RNA synthesis and repair, and cell membrane stabilization [1–6]. Zinc also regulates immunological processes, affecting hematopoiesis, lymphocyte maturation and differentiation and antibody production [4, 7–10]. It is a cofactor of over 300 enzymes involved in wound healing, a component of proteins including collagen and a regulator of genetic expression [7, 8, 10]. It is also involved in neurotransmission [11], affecting learning and memory processes [10]. In addition, it participates in maintaining normal glucose levels by sensitizing cells to insulin. Zinc also plays an important role in the synthesis of nitric oxide, which influences the relaxation of blood vessel muscles and contributes to their expansion.
Epidemiological studies have found low dietary zinc to be associated with an elevated cancer risk [5], being involved in the pathogenesis of neoplastic diseases such as lung cancer, pancreatic cancer, breast cancer and hepatocellular carcinoma [2, 9, 10, 12]. Zinc deficiency is also associated with malaria, tuberculosis, viral infections including HIV and HCV [2, 8, 9, 13], and the development of atherosclerosis, schizophrenia, depression, Alzheimer’s disease, multiple sclerosis, diabetes or Ehlers-Danlos syndrome [2, 9, 10, 14, 15].
For this reason, many people took zinc supplements during the SARS-CoV-2 pandemic; however, it has not been confirmed that the course of COVID-19 disease is milder in zinc-supplemented individuals [16, 17]. Nevertheless, it has been proven to be effective in treating childhood diarrhoea, Wilson’s disease, age-related macular degeneration [7, 9], and hepatic encephalopathy [12].
Metabolism
As the human body is unable to synthesize zinc, it is necessary to provide the mineral in the diet or as pharmacological supplementation. It is estimated that the total zinc content in the human body is about 1.5–3 g [9, 10, 18, 19]. The highest tissue Zn concentrations are found in the skeletal muscle (about 60%), bone (about 30%), skin (about 5%, mostly in the epidermis) and liver (about 5%) [2, 4, 19]. There is also a relatively small amount of Zn in blood serum (about 0.1% of the total zinc stores) [13, 20, 21]. However, the human body does not have a specific store for the element [2, 3, 6, 18, 22]. Transcriptional mechanisms and transmembrane transport modulate zinc homeostasis, and these protect the body from the effects of excess dietary zinc, or its deficiency, to some extent [4, 8]. It is necessary to take in optimal doses of zinc, adequate for daily requirements, to maintain its homeostasis [6, 10]. Zinc is absorbed in the duodenum and proximal jejunum, while it is excreted mostly in the faeces, and to a lesser extent through renal filtration and surface losses [3, 7, 13, 18]. The rate of absorption is concentration dependent, and rises with increasing dietary zinc content [13]. The total pool of the element in the body also affects intestinal absorption, which is enhanced in zinc-deficient individuals [13].
Assessment of zinc stores in the body
Blood serum analysis
In clinical practice, the most commonly used Zn assay is serum Zn concentration. Normal values are assumed to be within the range of 80–100 µg/dl (12–15 µmol/l) [23]. Although serum testing is technically easy, the interpretation of the results has some limitations [7, 13, 21, 24–26]. Intracellular zinc ion content may be low despite normal serum values. This is due to the possibility of Zn release from cells in states of severe deficiency [24, 27]. On the other hand, low blood zinc content does not necessarily indicate a general deficiency, but only reflects a physiological response to the inflammatory processes taking place in the organism [13, 20, 28]. For example, during the acute phase of the immune response, zinc is transported from the serum to the liver, leading to hypozincemia [9, 13, 15, 27]. The resolution of inflammation leads to the release of Zn from tissues into the blood [9].
In serum, zinc binds to albumin, α2-macroglobulin and transferrin [8, 9]. It is estimated that free Zn2+ ions in serum account for only about 0.0001% of the total body Zn stores [8, 10]; albumins bind approximately 70–80% of the pool of zinc circulating in the blood [13, 29, 30].
Serum zinc levels are reduced during hypoalbuminemia [13, 20, 22], through inter alia increased urinary excretion [12, 29]. A similar effect occurs during hormonal treatments, including glucocorticosteroids, when Zn is transported into cells [13, 27].
Zinc concentrations increase as a result of intravascular and extravascular haemolysis following its release from erythrocytes [20, 27, 31]. Serum Zn levels also demonstrate as much as 20% diurnal variability [13, 23]. In addition, zincemia rapidly increases after a meal, before decreasing after 2–4 h [25]; following this, serum Zn concentration gradually increases until the next meal [13]. Furthermore, the highest blood serum Zn values are found in the morning, after several hours of fasting [13, 23].
Urine analysis
To determine the zinc content in urine, it is necessary to perform a 24-hour urine collection. It is assumed that the daily amount of zinc excreted in urine should be within the range of 300–600 µg, i.e. 4.6–9.2 µmol/24 h. Urine zinc levels may vary over the day and these depend on both diuresis and the amount of Zn in the diet. Hyperzincuria may occur due to high dietary zinc supply or poor absorption in the small intestine, which in turn may be due to alcoholism, cirrhosis, acute porphyria, type II diabetes mellitus, lead poisoning, proteinuria, and treatment with chelating compounds [31]. Starvation, intense exercise and trauma can also increase urinary zinc elimination [32]. In contrast, in most zinc-deficient individuals, urinary excretion is reduced in order to limit excessive loss [27, 33]. However, it should be noted that in conditions such as liver cirrhosis, chronic kidney disease or sickle cell anaemia, it is paradoxically hyperzincuria that presents as a sign of zinc deficiency [31]. Hence, determination of urinary Zn levels can sometimes be inconclusive and difficult to interpret [22, 33].
Hair analysis
Hair is another substrate that can potentially be used to assess zinc content in the human body. Sample collection is pain free and non-invasive [28, 34]. Hair Zn concentration accurately reflects tissue zinc stores, and can present a long-term picture of zinc content for up to several months prior to sample collection [28, 33, 34]. Therefore, it is not suitable for a comprehensive assessment of a patient’s current zinc status. In addition, hair zinc content is also influenced by factors such as age, gender, hair growth rate and season [28, 32]. Furthermore, in conditions of retarded hair growth, e.g. in malnutrition or severe zinc deficiency, Zn hair concentration may paradoxically be normal or even high [11]. Therefore, despite its advantages, no standardized procedures have been developed for hair collection, preparation and analysis [32], and its clinical usefulness and reference values remain unclear [20, 26, 32, 35, 36].
Zinc deficiency
It is estimated that zinc deficiency may affect up to 17% of people worldwide [8, 10], most of whom being from Africa or Asia [8]. Zn deficiency is rarely diagnosed in developed countries, and is mainly observed in elderly people with multiple comorbidities, especially autoimmune or inflammatory diseases [8, 9, 37]. The condition can be caused by increased demand, decreased supply, impaired absorption, or excessive loss [3, 10, 27]. The highest demand for Zn occurs in pregnant and lactating women, in children and in the elderly [3, 13]. Zn deficiency may also result from a deficient diet, with limited or no animal protein, in malnourished individuals, and in those with inflammatory bowel diseases or cirrhosis [7, 8, 10]. Chronic diarrhoea, parenteral nutrition or alcohol abuse may also contribute to the development of zinc deficiency [7, 8].
Mild zinc deficiency
The possible symptoms of zinc deficiency are presented in Table 1. Mild and moderate zinc deficiency is characterized by non-specific symptoms: roughened skin, alopecia, nail dystrophy, loss of appetite, impaired wound healing, taste and smell disorders or photophobia [3, 7, 20, 27, 38, 39]. Zinc-deficient individuals demonstrate increased susceptibility to infections associated with lymphopenia, abnormal lymphocyte function, thymic atrophy and oxidative stress [3, 6, 8, 9]. In children, Zn deficiency is associated with growth retardation and delayed puberty [3, 20, 38].
Table 1
Symptoms of zinc deficiency
Acrodermatitis enteropathica
Acrodermatitis enteropathica (AE) is a disease associated with a severe zinc deficiency caused by a genetic defect in the cellular transporter ZIP4 (Zrt/Irt-like protein 4), which is responsible for intestinal absorption [2, 4, 9]. If undiagnosed and untreated, AE can lead to death [3, 4, 6, 9]. The condition is inherited in an autosomal recessive pattern. Its incidence is estimated at about 1 in 500,000 children [19]. AE manifests soon after the cessation of breastfeeding, or earlier if the child is fed with formula milk [33, 39]: breast milk generally provides a sufficient source of zinc for the first 6 months of life [33].
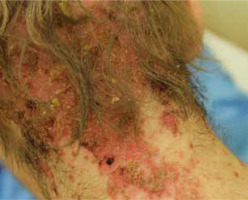
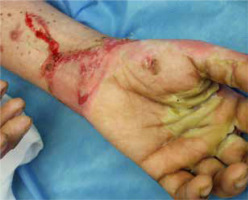
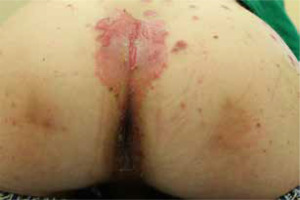
The most common symptoms in children are diarrhoea, weight loss and recurrent infections [13, 26]. Skin lesions often appear later in the course of the disease [13]. In most cases, the lesions are erythematous, pustular or bullous [3, 20, 33, 38], and because of scaling, they might resemble psoriatic plaques [20, 33, 39] (Figure 1). Erosions or non-healing ulcers may also be present [13, 38] (Figures 2, 3). The skin lesions are sharply demarcated from the surrounding skin [33]. Signs of bacterial and/or yeast infection are also frequently observed. Typically, the skin lesions are centred around natural body orifices (anogenital, perioral region) and on acral areas [18, 19, 20, 26, 33, 38, 39] suggesting that their development may be influenced by irritants such as saliva, urine, faeces or external factors [19, 38]. It has been found that zinc-deficient individuals demonstrate lower numbers of Langerhans cells in the skin, due to a decrease in tumor growth factor-β1 expression [10, 19]; this causes an abnormal release of adenosine triphosphate from keratinocytes in response to external irritants, resulting in the development of irritant contact dermatitis [19].
Diffuse telogen effluvium and nail dystrophy are also common in AE [3, 7, 19]. It has been shown that patients with telogen effluvium not suffering from AE also have lower serum zinc concentrations compared to healthy individuals, and zinc supplementation enables hair to regrow [19]. Presumably, as a result of Zn deficiency, the process of keratinization in hair is disturbed and the telogen phase occurs prematurely [19]. However, the exact mechanisms of this phenomenon have not been established [19].
The histopathological examination of the skin lesions reveals non-specific features which are also characteristic of necrotic migratory erythema or vitamin B3 deficiency [19, 33], with common findings being ballooning degeneration of keratinocytes, parakeratosis, thick chromatin aggregates, acanthosis, focal acantholysis, decreased stratum granulosum and increased mitosis [19, 33, 40, 41]. Dilated capillaries and lymphohistiocytic infiltration in the papillary dermis may also be present [33, 41].
Zinc is a key element for the proper synthesis, storage and secretion of male sex hormones [10]. In men, Zn deficiency can lead to symptoms of hypogonadism and impaired spermatogenesis [3, 7, 10, 20]. In addition, cognitive decline, memory and behavioural disorders are sometimes observed in patients of both sexes [10, 13].
The basic treatment of acquired zinc deficiency is Zn supplementation in the dosage of 0.5–1 mg/kg b.w./day [9, 20]. In most patients, a few months’ therapy is sufficient to achieve optimal Zn levels. However, in acrodermatitis enteropathica, patients require higher doses of zinc (3 mg/kg b.w./day) and the supplementation must be maintained throughout life [20, 33, 39].
Zinc and chronic leg ulcers
Ulceration is, by definition, the loss of the full thickness of skin [42]. It is estimated that chronic venous insufficiency may account for 50–80% of all leg ulcers, occurring in 0.3–1% of the population in Western countries, and even more often in the elderly [25, 42–44]. Leg ulcerations also commonly arise in association with arterial diseases, diabetes and pressure [44, 45]. Healing time is approximately 6 to 12 months, and the majority of patients (50–70%) have recurrent lesions [43, 44, 46, 47]. The accompanying pain and reduced mobility leads to a significantly reduced quality of life.
Many zinc-dependent proteins, such as metallothioneins, metalloproteinases, integrins, alkaline phosphatase and transcription factors, are involved in wound healing [4, 19, 45]. Wound healing is a multistep process consisting of inflammation, proliferation, angiogenesis, epithelialization, wound contraction and remodelling [4, 45]. Zinc regulates inflammation, accelerates epidermis restoration and collagen synthesis in the ulcer, and stimulates fibroblast and keratinocyte proliferation [1, 10, 48].
Many studies report reduced serum zinc levels in patients with chronic ulcers [49–51]. In the 1970s, Henzel et al. observed that patients with significantly slower healing of postoperative wounds showed lower levels of zinc in serum, wound margins and granulation tissue [52].
However, there is still insufficient evidence to suggest that oral Zn supplementation or topical preparations improve healing of ulcerations, especially in patients with normal Zn levels [4, 7, 33, 53]. A 2014 meta-analysis of six studies reached no clear conclusions regarding the usefulness of zinc supplementation in ulcer healing; still, it should be emphasized that the authors only analysed a small number of studies, conducted many years ago on relatively small groups [25].
Zinc in the pathogenesis of other dermatoses
It is likely that zinc deficiency also plays a role in the pathogenesis of other skin diseases by increasing oxidative stress and influencing the immune system [54]. Significantly lower serum and hair Zn concentrations have been noted in patients with atopic dermatitis (AD) compared to healthy populations [28, 55–59]. However, there is no consensus whether zinc supplementation reduces the severity of clinical symptoms of AD, or the need for oral and topical medications [28, 55, 58–61]. Similarly, lower serum Zn levels have been found in patients with seborrheic dermatitis [54], Behcet’s disease [62], acne vulgaris [63], melasma [64], alopecia areata [65], and oral lichen planus [66]. Interestingly, patients with erosive oral lichen planus demonstrated lower mean serum Zn levels than those with the non-erosive type [66]. Zinc deficiency has also been observed in bullous pemphigoid [65], epidermolysis bullosa [67] and pemphigus vulgaris [68].
Excess zinc
Due to the low toxicity of zinc, especially when administered orally, overdose is relatively rare [14, 27]. Excess Zn may cause nausea, vomiting, headache, dizziness and fatigue, and it may result in anaemia, neutropenia or decreased levels of high-density lipoproteins if the recommended dose is significantly exceeded [3, 7, 20, 27, 28]. Paradoxically, impaired immune function may be a consequence of both Zn deficiency and excess [5, 9]. Chronic high zinc intake may also result in a copper deficiency as it impairs copper absorption in the gastrointestinal tract [8, 18, 28]. In individuals with optimal body Zn concentrations, 40 mg/day is considered the upper limit of zinc intake tolerated by adults [10, 35].
Supplementation and diet
The recommended daily intake of zinc by healthy individuals is not clearly determined. However, the World Health Organization (WHO) recommends values of 3–14 mg/day in adults based on age, sex and dietary phytate content [2]. The US Institute of Medicine recommends a zinc intake of 8 mg/day for women and 11 mg/day for men [10, 35, 39].
The most abundant sources of dietary zinc include fish and seafood (especially oysters), red meat, poultry, legumes, pumpkin and sunflower seeds, eggs, dairy products, and nuts [2, 7, 9, 10, 39]. The form of zinc intake and the dietary composition play a very important role as a meal has much lower zinc bioavailability than water-based solution [8, 11, 13]. On the other hand, the amount of protein in a meal is positively correlated with zinc absorption, with the exception of the milk protein casein [13, 69]. In addition, as Zn from animal products is absorbed more efficiently, vegetarians and vegans are more likely to be zinc deficient [2, 13, 20, 39].
Phytates are phytic acid salts and an important storage of phosphates and minerals [13, 70]. As chelators of zinc ions, they limit its absorption from the gastrointestinal tract, forming complexes that are difficult to dissolve [8, 9, 13, 69, 70]. Legume seeds and cereals are products rich in phytates [8, 13]. Whole grain products are particularly rich in phytates, which is found in the aleurone layer and the germ [70]. However, milling, thermal processing, leavening of bread, germination, and fermentation reduce the adverse effects of phytates, thus improving zinc bioavailability [13, 69].
High doses of iron (Fe), especially in water-based solution, can interfere with zinc absorption if administered simultaneously and with a high Fe/Zn ratio [69]. No such effect is observed for the iron contained in foods [13]. Thus, it seems that even long-term use of iron supplements does not affect zinc absorption, given appropriate doses and intervals in their intake [69].
Calcium supplements and calcium-fortified foods may also have a negative impact on zinc absorption [70]. However, the mechanism is complex and probably not associated with calcium itself, rather than the potential augmentation of the inhibitory impact of phytates [13].
Zinc in topical formulations
Topical formulations containing Zn, mainly in the form of 1–15% zinc oxide or sulfate, are commonly used to support the treatment of ulcers, acne vulgaris, rosacea or diaper rash [7, 39]. It is also used as an ingredient in sunscreens as zinc oxide provides broad-spectrum protection against UV radiation [39]. In high concentrations (20–25%), zinc sulfate has cytotoxic effects, inducing cell apoptosis and necrosis, which is valuable in treatment of precancerous conditions (solar keratosis, xeroderma pigmentosum) and basal cell carcinoma [39].
Topically-applied zinc acts as an astringent, debriding and antiseptic agent [3, 40]. Such formulations create a barrier film, protecting the wound from exudation, maceration and irritation of the surrounding skin [71]. However, zinc formulations are not transparent, thus the wound margin is partially obscured, making the observation of the epithelialization process difficult [71]. Local hypersensitivity reactions to zinc oxide are rare, although possible [40, 71].
Commercially available zinc paste-impregnated bandages can be used in patients with chronic venous insufficiency, and those with ulcerations [40]. These form semi-rigid dressings, which can also act as a type of compression therapy (the so-called Unna boot) [71]. Nowadays, dressings made of elastic bandages are easier to use, better fitted and provide sustained pressure, and are hence in more common use [71]. However, non-elastic materials demonstrate better hemodynamic efficiency, and thus greater elimination of venous hypertension, reduction of swelling, and better healing conditions; as such, the Polish Dermatological Society recommendations indicate a preference for zinc paste dressings in the treatment of venous ulcers at the healing stage [72].
Summary
The first reports on the important role of zinc in physiological processes date back to the early 20th century, when its content in human tissues was first determined. Despite its relatively low concentrations, Zn is known to have a considerable influence on the functioning of the human body. A number of recent studies examining the optimal concentration of zinc in serum and the mechanisms of its systemic and local effects have demonstrated the usefulness of zinc supplementation in many diseases. However, a number of questions regarding the potential use of zinc in the treatment of skin diseases still remain open.