Purpose
Brachytherapy (BT) is a validated radiation technique for early stage tumors of the oral cavity and oropharynx combined with surgery and/or external beam radiotherapy (EBRT), depending on the indication. It enables targeted irradiation of the tumor or surgical bed with a high-dose gradient, consequently sparing organs at risk (OARs). No randomized trial has compared brachytherapy with other techniques, but results obtained in large series of patients validated BT treatment for localized tumors [1, 2]. Brachytherapy of head and neck cancers has been formerly performed using iridium wires with low-dose-rate (LDR) BT, but is progressively being replaced by pulse-dose-rate (PDR) BT, with comparable results [3]. The aim of this study was to analyze the results of patients treated in our institute from 2009 to 2020 after replacing LDR-BT with PDR-BT.
Material and methods
We retrospectively collected data from patients treated between 2009 and 2020 for squamous cell carcinoma (floor of the mouth, tongue, or oropharynx) using adjuvant interstitial BT with or without EBRT. Patients with a follow-up of less than one year, patients with re-irradiation, and those with lip tumors were excluded. Indications for BT after surgery for the tongue, floor of the mouth, and tonsil tumors were T1-T2 N0 tumors with borderline margins, without lympho-vascular invasion (LVI), or perineural invasion (PI). Indications for radiotherapy (RT) with BT boost after surgery were T1-T2 tumors of the tongue and floor of the mouth with positive nodes, LVI, or PI. Data collected included age, tumor characteristics (i.e., location, size, and TNM using the 8th AJCC classification), and associated treatments (i.e., surgery or surgery plus EBRT). Also, margin status, presence of LVI or PI, time between surgery and EBRT or BT, dose, and overall treatment time were recorded. Regarding characteristics of BT, the following were recorded: number of vectors, spacing between vectors, dose, dose-rate, coverage index (CI), dose homogeneity index (DHI), overdose volume index (OI), dose non-uniformity ratio (DNR), conformity index (COIN) [4], prescription isodose volume, and total dose (EQD2) in BT or EBRT + BT. After BT, patients underwent a clinical examination with naso-fibroscopy at one month, every 3 months for the first 2 years, and then every 6 months for 3 years. Computed tomography (CT) scan was performed 3 months after BT treatment and in case of clinical signs. The primary outcome was local control. The secondary outcomes were regional control and toxicities (i.e., mucositis, pain, osteoradionecrosis [ORN], soft tissue necrosis [STN]) defined using CT CAE v. 4 scale [5] as well as their duration and management.
Statistical analysis
Results were expressed as median and interquartile range (IQR) for quantitative variables, and as numbers and percentages for qualitative variables. Local recurrence (LR) was defined as the time between BT and the date of local recurrence. Regional recurrence (RR) was specified as the time between BT and the date of RR. The incidence of toxicity was defined as the time elapsed between the date of BT administration and the appearance of specific toxicity. Local and regional recurrences were described using Kaplan-Meier method. Occurrence of toxicity was evaluated with cumulative incidence method, considering recurrence and death as competing risks. Risk factors for recurrence were evaluated using univariate Cox model, and those for the incidence of toxicity were assessed with univariate analysis using Fine-Gray model. Results were presented as adjusted hazard ratios (HRs) with a 95% confidence interval (CI). Statistical significance was defined as p < 0.05. All analyses were performed using R Studio software, version 2022.07.2+576.
Results
Patient characteristics
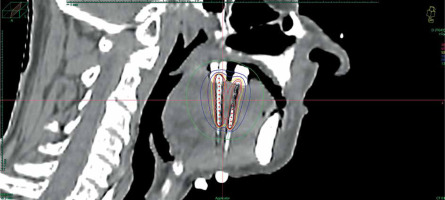
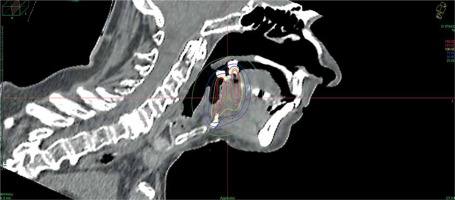
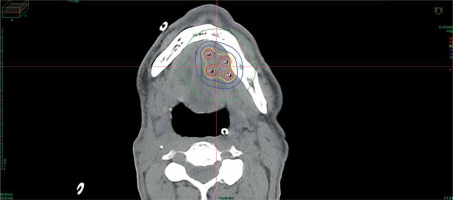
Data of 66 patients were collected between 2009 and 2020. Patient tumor and treatment characteristics are shown in Table 1. Figures 1-3 show examples of brachytherapy. Anatomopathological analysis revealed millimetric or infra-millimetric margins in 53.1% of cases, LVI in 15%, and PI in 30%. The median follow-up was 48.87 months (range, 22.32-71.45 months).
Table 1
Patient characteristics
Implantation characteristics and dosimetry
Number of vectors, vector spacing, and prescription isodose volume are reported in Table 2 for all patients, except for missing data on prescription isodose volume for one patient. EQD2 was higher for patients treated with EBRT + BT, and dose-rate was higher for patients treated with BT only.
Table 2
Implantation characteristics
Local and regional control
Local and regional recurrences were reported in 11% and 20% of patients, respectively. No significant factors were identified (Table 3). Of the six patients with isolated local recurrence, only two underwent surgery. The first patient had no second recurrence, and the second had a regional recurrence treated with radiochemotherapy. The remaining four patients were not amenable to surgery, received palliative chemotherapy, and died. Of the patients with regional recurrence, six were treated with surgery, followed by radiotherapy or radiochemotherapy. One of these patients experienced a second recurrence, received palliative chemotherapy, and died. One patient with regional recurrence was treated with radiochemotherapy without recurrence, and another patient was treated with palliative chemotherapy and died.
Table 3
Relationship between recurrence, tumor, and treatment characteristics
Toxicities
Grade 2 and 3 acute mucositis was reported in 21% (n = 14) of the patients (18% grade 2, and 3% grade 3). Almost half (47%, n = 32) of the patients described acute pain following BT, and 26% (n = 17) required stage 2 or 3 analgesics. Chronic toxicities observed were STN and ORN. Trophic disorders were observed in 16 (24%) patients. Five patients (7.6%) presented with STN, and required treatment with tocopherol and torental, of whom 1 subsequently required hyperbaric oxygen therapy (5 stage 2, and 1 stage 3). No predictive factors were identified for STN risk (Table 4). Two patients had ORN (stage 3) at 19 and 91 months after BT. Grade 2 and 3 acute mucositis were more frequent with BT than with EBRT + BT (HR = 0.13, p = 0.040). There was no difference in terms of pain severity, analgesic treatment, or late toxicity between the BT and EBRT + BT groups.
Discussion
This retrospective study included a large series of PDR-treated head and neck tumors at a single experimental institute.
Comparison of results with literature (Table 5)
In terms of local control and toxicities, the results of this cohort study were similar to those described in the literature on PDR and LDR. With LC of 87% and RC of 82% at 5 years, our study obtained results comparable with 2 series of patients with LDR-BT published in 1995 and 2014 by our institution (LC: 89% and 97%, RC: 82%) [3, 6]. Consistent series of 36-236 patients treated with PDR-BT presented an LC rate between 88% and 97% at 3-5 years [3, 7, 8]. With an STN rate of 8% and ORN rate of 2.4%, late toxicities were lower than those reported in LDR studies (STN: 12% and 7%; ORN: 6% and 5%), and comparable with those of the previously cited PDR studies (STN: 8% to 11%, ORN: 3% to 7.6%).
Compared with BT-HDR series, in terms of LC, our study achieved comparable results (LC: 53-87% at 2-5 years) [9-14]. In terms of toxicity, the results of this series are superior to those of the oldest HDR series (SNT: 15-35%), with doses per fraction of 3-6 Gy [9-12]. More recent studies with fractional doses of 3 to 4 Gy have shown comparable results (SNT: 9.3-16%, ORN: 2-4%) [13, 14].
Risk factors for toxicity
Acute grade 2 and 3 mucositis were more frequent in the BT group than in the EBRT + BT group, despite a lower EQD2 on the tumor. This result is not surprising given that the treatment is concentrated over five days in BT and not several weeks in EBRT + BT. The BT group showed a significantly higher dose-rate compared with the EBRT + BT group in order to be able to carry out the treatment over 5 days and avoid removal of the vectors on weekends. Indeed, for safety reasons in the event of bleeding, equipment removal is performed on working days when all personnel (brachytherapist, surgeon, anesthetist, etc.) are on site. Therefore, dose-rate was sometimes increased, but generally it remained within the recommended thresholds. As BT treatment time in addition to EBRT was shorter, it was not necessary to increase flow rate in this group to avoid vector ablation on weekends. However, there was no difference between the two groups in terms of pain, extent of analgesic treatment, or late toxicity. The two groups presented different tumor stages (more advanced stage and N+ for the EBRT + BT group). Moreover, no statistically significant risk factors for toxicity were identified in the present study. The known risk factors in the literature include vector spacing, treated volume, dose-rate, and location [1, 15]. However, in the current study, these elements were in line with the ESTRO GEC recommendations [1], which aim to limit toxicity. The median dose-rate was 50 cGy per hour; however, 13 patients had a rate exceeding 60 cGy per hour, although the recommended rate was between 30 and 60 cGy/h. The vector spacing was between 1 and 1.5 cm. The median volume receiving prescribed dose was 22 cc, and the maximum number of vectors was 6. In a series reported by Pernot with 1,134 patients, the authors concluded that there was an increase in toxicity above 30 cc and 6 vectors [15]. A location close to the mandible and the absence of lead protection are known risk factors for ORN [1]. The two patients who developed ORN were checked to see if they wore their lead shields during treatment. The distance between the osteoradionecrosis site and the nearest brachytherapy vector was 2 mm and 4 mm for these patients. However, both were treated for cancer of the floor of the mouth, which is the location closest to the mandible, considered the most at risk in the literature.
Dose-rate
The recommended BT technique for VADS tumors is low-dose-rate, with a significant number of series and patients [3, 6-8, 15, 16]. Haddad’s single-center study in our institution showed no difference in local control or toxicity between LDR and PDR (LC: 97% vs. 94%, STN: 7% vs. 8%, and 7% vs. 3%) [3]. Other PDR series, including ours, have shown similar results to those of LDR [7, 8]. However, for other sites, there is a transition to a HDR-BT for economic reasons and to alleviate constraints, including medical on-call duty, all-day and all-night patient monitoring, supervising of technical developments with manufacturers, and radioprotection. The first published study on HDR-BT for oral or oropharyngeal cancers were very heterogeneous in terms of fractionation and dose (Table 5) [9-12]. The toxicity in some reports appeared to be higher than those in PDR. For example, Nose’s series of 83 patients showed 83% local control and 29% STN with 6 Gy per fraction [12]. In 2009, the ESTRO GEC recommended no more than 4 Gy per fraction for oral cavities, and most series with high toxicities proposed higher doses per fraction [2]. More recent study by Guinot and Santos, including 50 and 43 patients who received treatment to the tongue, with doses between 3 and 4 Gy per fraction, found late toxicity rates closer to PDR series [13, 14]. A meta-analysis of six oral cavity BT trials, including 607 patients (LDR: 447 and HDR: 160), showed no differences in terms of local control, survival, or toxicity (grade 3 and 4) [17]. Brachytherapy PDR and LDR series were used as a reference to verify the toxicity of HDR-BT and to adapt the dose if necessary. We have moved from PDR to HDR, and this study will serve as a baseline for future studies on HDR brachytherapy.
Table 5
Clinical comparative outcomes and toxicities from oral and oropharyngeal brachytherapy studies on low-dose-rate (LDR), pulse-dose-rate (PDR), and high-dose-rate (HDR)
| Author [Ref.] | Year | No. of patients | Dose-rate | Location | EBRT + BT or BT | LC (%) | RC (%) | STN (%) | ORN (%) | Median follow-up time (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pernot [6] | 1995 | 97 | LDR | Oral | EBRT + BT or BT | 89 | 82 | 12 | 6 | 5 |
| Lau [9] | 1996 | 27 | HDR (6.5 Gy/fx.) | Oral | BT | 53 | NC | 35 | NC | 5 |
| Levendag [10] | 1997 | 15 | HDR (3-5 Gy/fx.) | Oropharyngeal | EBRT + BT or BT | 87 | NC | 30 | NC | 3 |
| Inoue [11] | 2001 | 25 | HDR (6 Gy/fx.) | Oral | BT | 87 | NC | 15 | NC | 3 |
| Nose [12] | 2004 | 83 | HDR (6 Gy/fx.) | Oropharyngeal | EBRT + BT or BT | 83 | NC | 29 | NC | 2 |
| Strnad [7] | 2005 | 236 | PDR | Oral and oropharyngeal | EBRT + BT or BT | 88 | NC | 9.7 | 7.2 | 5 |
| Melzner [8] | 2007 | 210 | PDR | Oral and oropharyngeal | EBRT + BT or EBRT | 93 | NC | 11 | 7.6 | 2 |
| Guinot [13] | 2010 | 50 | HDR (3-4 Gy/fx.) | Oral | EBRT + BT or BT | 79 | NC | 16 | 4 | 5 |
| Haddad [3] | 2014 | 36 72 | PDR LDR | Oral and oropharyngeal | EBRT + BT or BT | 94 97 | NC | 8 7 | 3 7 | 3 5 |
| Santos [14] | 2022 | 43 | HDR (4 Gy/fx.) | Oral | EBRT + BT or BT | 84 | 81 | 9.3 | 2 | 5 |
| Present study | 2023 | 66 | PDR | Oral and oropharyngeal | EBRT + BT or BT | 87 | 82 | 8 | 2.4 | 4 |