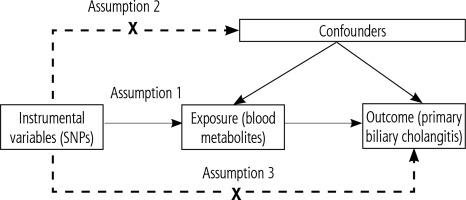
Introduction
Primary biliary cholangitis (PBC), also known as primary biliary cirrhosis, is a chronic inflammatory liver disease characterized by autoimmune-mediated progressive bile duct obstruction. It predominantly affects middle-aged to elderly women and individuals with non-suppurative granulomatous lymphoplasmacytic cholangitis [1].
The pathogenesis of PBC may involve a complex and chronic cascade reaction, where the biliary epithelial cells interact with immune metabolites and bile acids. The detailed mechanisms underpinning these processes are not fully delineated. With enhancements in diagnostic methods and increasing disease awareness, the epidemiology of PBC is progressively changing. The diagnostic rate increased from 41% in the 1970s to 72% in the 1990s [2]. The global incidence of PBC continued to rise before 2000, after which it stabilized [3]. Research indicates that from 2011 to 2020, the incidence rates in the Asia-Pacific region, Europe, and North America changed from 0.60, 1.91, and 3.51 to 0.86, 2.61, and 2.75, respectively [4]. Previous studies have shown that in the 1990s and early 2000s, the ratio of female to male patients with PBC was 9 : 1 [5]. Recent studies indicate that the aforementioned ratio has decreased; however, the predominance of females remains a key characteristic of PBC [3]. In European and Asian countries, the prevalence and incidence of PBC in women are still 5 times and 4 times higher, respectively, than in men [6]. Currently, expert panels agree that the diagnosis of PBC is supported by meeting at least two of the following three criteria [7]: Scenario 1: Chronic elevation of alkaline phosphatase (ALP) with a positive anti-mitochondrial antibodies (AMA; immunofluorescence assay titer of > 1 : 40 or enzyme immunoassay > 25 units) in the absence of other liver and systemic diseases. Scenario 2: Chronic elevation of ALP with negative AMA but positive PBC-specific antinuclear antibody (ANA) (sp-100, gp-210) tests or a reticular pattern of ANA. Scenario 3: Chronic elevation of ALP with negative AMA and ANA tests, but a liver biopsy showing nonsuppurative cholangitis and destruction of the interlobular bile ducts. Untreated PBC patients typically progress to cirrhosis and hepatic failure within 10-20 years. Drug development endeavors for PBC are ongoing, targeting pathways involved in bile duct obstruction, anti-fibrosis, immune regulation, and metabolic regulation, yet a cure-all treatment remains elusive [8].
As the liver undergoes immune responses and damage, a diverse array of plasma metabolites becomes pronounced, reflecting the pathological and physiological alteration within [1]. Metabolites in the serum, as functional intermediates under environmental exposure (including chemicals, heavy metals, pollutants, drugs, nutrients, microorganisms, and radiation, and more) [9], can induce permanent changes in cellular tissues and genetic structures through epigenomic modulation [10]. Metabolomic studies in liver diseases are commonly associated with conditions such as cirrhosis and liver cancer, but research on serum metabolites related to PBC, an autoimmune liver disease, is relatively rare. Hao et al. [11] established that the metabolism of serum glycine, serine, and threonine is associated with the progression of PBC. Shayesteh et al. [12] found that hepatic and cardiac kynurenine/tryptophan ratios were elevated in rats with cholestatic liver cirrhosis and were reduced following 1-methyl tryptophan (1-MT) administration. Tang et al. [13] compared small molecule metabolites in the blood of PBC patients and healthy controls and found that bile acid levels increased with the progression of PBC, while propionyl carnitine and butyryl carnitine decreased. Many studies from human, cellular, and animal models indicate a connection between serum metabolites and PBC [11-13]. However, the underlying mechanisms remain unclear, and traditional observational studies grapple with the challenge of establishing a definitive causal relationship between serum metabolites and the emergence of PBC.
Mendelian randomization (MR), a technique frequently utilized in genetic epidemiology, leverages genetic variation as an instrumental variable to investigate causal relationships between exposome factors and clinical outcomes [14]. Large-scale genome-wide association studies (GWAS) analysis provides the possibility of genetically screening pathogenic plasma metabolites related to PBC [15]. Therefore, this study employed a two-sample MR analysis to assess the causal relationship between 1,400 plasma metabolites and PBC. Through this approach, our study aimed to identify important biomarkers associated with PBC that may play a crucial role in the early diagnosis and management of the disease. Additionally, clarifying the causal relationships between these metabolites and PBC will provide a scientific basis for developing targeted intervention strategies in the future, thereby helping to improve treatment outcomes and quality of life for patients.
Material and methods
Study design
Based on a two-sample MR analysis, we assessed the causal relationship between 1,400 plasma metabolites and PBC. MR utilizes genetic variation as a proxy for risk factors. Therefore, instrumental variables in causal inference must satisfy three key assumptions: 1) genetic variation is directly associated with exposure; 2) genetic variation is unrelated to potential confounders between exposure and outcome; 3) genetic variation does not affect the outcome through pathways other than exposure (Fig. 1).
Genome-wide association study data sources for primary biliary cholangitis
The GWAS data for PBC are derived from a meta-analysis conducted by Cordell et al. [16], published in Nature Communications. The study utilized samples from 2,764 PBC patients and 10,475 healthy individuals of European descent. Approximately 1.12 million single nucleotide polymorphisms (SNPs) were employed for association analysis. The data are publicly accessible in the GWAS catalog (Trait: Primary biliary cholangitis – IEU OpenGWAS project (mrcieu.ac.uk)) with the accession number ebi-a-GCST003129.
GWAS data for 1,400 plasma metabolites
The GWAS data for plasma metabolites originate from a study conducted by Chen et al. [17], published in Nature Genetics (Supplementary Material 1). The data are publicly available in the GWAS catalog (GWAS Catalog (ebi.ac.uk)) with the accession number GCST90199621-90201020. The study comprises meta-analysis data from 8,299 individuals of European descent, involving 1,091 plasma metabolites and 309 metabolite ratios for GWAS. Among the 1,091 plasma metabolites, 850 are known and categorized into 8 classes: carbohydrates, amino acids, nucleotides, cofactors and vitamins, lipids, peptides, energy products, and xenobiotics. The remaining 241 are classified as unknown or “partially” characterized molecules.
Selection of instrumental variables
To meet the assumptions, we rigorously identified instrumental variables (IVs) associated with plasma metabolites from multiple perspectives using strict screening criteria. Given a moderate number of SNPs related to plasma metabolites, we selected SNPs associated with plasma metabolites based on a genome-wide significance level (p < 1 × 10-5). To ensure that the instrumental variables are genetically independent, we focused on SNPs by removing linkage disequilibrium (LD, R 2 > 0.001 within 10,000 kb). To mitigate bias, we calculated the F-statistic for each SNP to measure its strength as an instrumental variable, considering SNPs with F < 10 as weak instrumental variables and excluding them [18]. We used the PhenoScanner database to examine SNPs potentially associated with confounding factors, and those that could violate the independence assumption were excluded. After rigorous screening, the remaining SNPs were considered qualified instrumental variables.
Mendelian randomization analysis
Under various assumptions, we applied several robust MR statistical methods to calculate the estimates of the impact of plasma metabolites on PBC. These methods include inverse variance weighting (IVW) [19], MR-Egger regression [20], the weighted median method (WM) [21], the simple mode [22], and the weighted mode [22]. The IVW method is the most frequently used approach in MR analysis [19], and it is the primary method employed in this study. MR-Egger, like IVW, incorporates an intercept term that assesses horizontal pleiotropy [23]. The p-value from a test of genetic pleiotropy is used to gauge the likelihood of pleiotropy in the analysis. If p > 0.05, it is considered that there is weaker evidence of genetic pleiotropy in causal analysis, and its potential impact can be disregarded. The WM method can produce correct estimates when less than half of the instrumental variables are invalid. Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) Analysis [24] is employed to detect horizontal pleiotropy and identify outlier SNPs. After removing these SNPs, the IVW method is re-applied to ensure that the results are not influenced by anomalous data. We utilized Cochran’s Q statistic and the corresponding p-value to test for heterogeneity among the selected IVs, where p > 0.05 indicates no heterogeneity. Sensitivity analysis was also conducted using the leave-one-out method [25], which evaluates whether MR conclusions depend on a specific SNP. If such a SNP is identified, its removal should be considered as part of the analysis.
In the IVW method analysis results, we applied a stringent multiple hypothesis testing threshold of p < 3.57 × 10-5 (p < 0.05/1,400) to assess significant causal relationships. The results can be categorized into three types: a direct causal relationship between plasma metabolites and PBC, a potential direct causal relationship, or the plasma metabolite being a potential risk factor. If the analysis results from all five models show p < 3.57 × 10-5, it is considered that there is a direct causal association between plasma metabolites and PBC. In the IVW method results, with p < 3.57 × 10-5, if any of the other four models shows 3.57 × 10-5 < p < 0.05, it is considered that there might be a direct causal relationship between plasma metabolites and PBC. If one of the analyses in both the IVW method and the other four models shows 3.57 × 10-5 < p < 0.05, it is considered that plasma metabolites could be a potential risk factor for PBC.
In summary, we rigorously screened plasma metabolites with potential causal effects on PBC using multiple criteria: 1) significant p-values from the initial analysis (p < 0.05 in IVW method), 2) consistency in direction and magnitude across the five MR methods, 3) absence of heterogeneity and horizontal pleiotropy in MR results, 4) minimal interference of MR estimates from individual SNPs.
This study also attempted to explore the causal relationship of PBC with plasma metabolites, by conducting a reverse MR analysis. We extracted significant and independent genetic SNPs from PBC GWAS data with a significance threshold of p < 5 × 10-8 and no linkage disequilibrium. Subsequently, we extracted outcome data information from the serum metabolites GWAS dataset and performed MR analysis following the methods mentioned earlier.
Statistical analysis and data visualization
All statistical analyses in this study were conducted using R and R Studio software, with R version 4.3.1. The “Two Sample MR” R package [26] and some foundational R packages were employed in the software. Forest and scatter plots were generated to illustrate the impact of each SNP on risk factors and outcomes.
Results
After the screening, six plasma metabolites out of the 1,400 were identified with potential causal associations with PBC. These metabolites include homoarginine, campesterol, sphingosine 1-phosphate, docosadienoate (22:2n6), g-glutamylglycine, and phenol sulfate. However, g-glutamylglycine was subsequently dismissed from the analysis due to inconsistent odds ratios obtained from its SNPs. Similarly, phenol sulfate was eliminated from consideration owing to its limited SNPs. In the end, we retained four plasma metabolites for further analysis, comprising a total of 20 SNPs.
Employing IVW as the primary analytical method, the investigation revealed that sphingosine 1-phosphate (OR = 0.65, 95% CI: 0.42-0.98, p = 0.04) and docosadienoate (22:2n6) (OR = 0.57, 95% CI: 0.36-0.90, p = 0.01) may confer a protective effect against PBC. Conversely, homoarginine (OR = 1.34, 95% CI: 1.04-1.72, p = 0.02) and campesterol (OR = 1.19, 95% CI: 1.01-1.40, p = 0.03) could be indicative of a heightened risk for developing PBC (Table 1).
Table 1
Causal relationships between blood metabolites and primary biliary cholangitis (PBC) based on inverse-variance weighted (IVW) method analysis
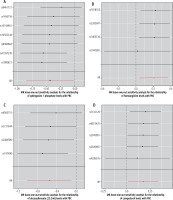
The heterogeneity test and genetic pleiotropy test were conducted for the above-mentioned four plasma metabolites. The outcomes, along with the results from five distinct MR models, are systematically presented in Table 2. Although not all five models achieved statistical significance, they exhibited similar effect sizes, possibly because the IVW method has higher testing efficiency than the other four MR models. The MR analysis results for the four plasma metabolites were visually summarized in scatter plots (Fig. 2). Furthermore, sensitivity analysis using the leave-one-out approach for the four plasma metabolites indicated that no individual SNP exerted a disproportionate impact on the collective results (Fig. 3).
Table 2
Causal relationships, heterogeneity tests, sensitivity analyses, and horizontal pleiotropy analyses for blood metabolites and primary biliary cholangitis (PBC)
Fig. 2
Scatter plots demonstrating causal effects in blood metabolites of primary biliary cholangitis (PBC): A) sphingosine 1-phosphate, B) homoarginine, C) docosadienoate (22:2n6), D) campesterol
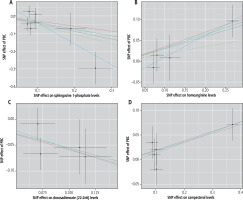
Fig. 3
Leave-one-out plots for the causal association between genetically predicted blood metabolites and primary biliary cholangitis (PBC). These blood metabolites include sphingosine 1-phosphate (A), homoarginine (B), docosadienoate (22:2n6) (C), and campesterol (D)
Expanding our analytical scope, we conducted a reverse MR analysis to examine whether PBC potentially influences the levels of the 1,400 plasma metabolites. Despite utilizing five distinct MR methodologies, no correlation was found between PBC and the plasma metabolites. The summarized results of MR, heterogeneity, pleiotropy, and sensitivity analyses for all methods regarding the genetically predicted PBC and plasma metabolites are provided in Supplementary Material 2.
Discussion
In this study, we utilized large-scale GWAS data from public databases and employed an unbiased two-sample Mendelian randomization analysis to investigate the causal relationship between an extensive panel of 1,400 plasma metabolites and the risk of PBC. After rigorous quality control, we identified four plasma metabolites that may have a causal relationship with the risk of developing PBC. Research into the metabolomics of serum is reshaping our understanding of disease mechanisms, offering insights into metabolic disruptions that may precede clinical manifestations. These metabolomic profiles are invaluable for early disease detection and differential diagnosis [27]. For instance, Chen et al. [28] identified six plasma metabolites through the analysis of ovarian cancer patients’ serum samples, suggesting their potential as biomarkers for early detection of ovarian cancer. Similarly, Barba et al. [29] found that plasma metabolomics could be used to predict exercise-induced ischemia in suspected coronary heart disease patients, implying that plasma metabolomics might assist in screening and risk stratification for individuals with coronary artery risk factors. Tan et al. [30] identified specific plasma metabolites that could aid in the early diagnosis of hepatocellular carcinoma (HCC), indicating the potential of plasma metabolomics in identifying biomarkers for liver cancer.
Recent findings illustrate that immune cells are highly sensitive to internal metabolites, rapidly adapting to microenvironments characterized by hypoglycemia and elevated lactate levels. Such adaptability is crucial for sustaining self-tolerance within the immune system and for regulation of the metabolic equilibrium [31, 32]. Yang et al. [33] suggested that serum glutamine and folate levels could potentially serve as specific biomarkers for PBC, with potential links to the immune damage mechanism and prognosis of PBC. Moreover, research spearheaded by Hao et al. [11] indicated that serum glycine, serine, and threonine metabolism is associated with the pathogenesis of PBC. Additionally, Shayesteh et al. [12] found an elevated tryptophan/serotonin ratio in the hepatic and cardiac tissues of rats with cholestatic cirrhosis. Another study noted increased conversion of tryptophan to serotonin in PBC patients, suggesting a correlation between serotonin metabolite levels and PBC severity [34].
In this study, two plasma metabolites, sphingosine 1-phosphate (S1P) and docosadienoate (22:2n6), emerged as being potentially protective against PBC. SIP is a crucial sphingolipid metabolite derived from intracellular sphinganine. Neural ceramidase converts sphinganine into sphingosine, which is then phosphorylated by sphingosine kinase (Sphk) to generate S1P [35]. S1P acts on specific G protein-coupled receptors, known as sphingosine 1-phosphate receptors (S1PRs), through either secretion or paracrine mechanisms, orchestrating a myriad of cellular processes including cell proliferation, migration, and angiogenesis [36]. The SIP/S1PR signaling axis also plays a role in the migration and differentiation of immune cells such as T cells, B cells, and dendritic cells, making it a potential target for immune-mediated diseases such as rheumatoid arthritis, systemic lupus erythematosus, atopic dermatitis, and inflammatory bowel disease [37]. Zhang et al. [38] found that in mice with autoimmune hepatitis, the use of S1P1 modulators significantly reduced serum liver function indicators (ALT, AST, ALP, etc.) and immunoglobulin (Ig) G levels. This had a pronounced alleviating effect on liver damage and inflammatory infiltration, leading to a decrease in levels of inflammatory factors such as interleukin (IL)-6 and tumor necrosis factor α (TNF-α) in both serum and liver tissues. Additionally, it reduced the T-cell content in peripheral blood, spleen, and liver, while increasing the T-cell count in the thymus and mesenteric lymph nodes, promoting T-cell homing. This offers new insights for the clinical treatment of autoimmune hepatitis. Given that PBC is another autoimmune hepatic condition, our findings revealed that serum S1P levels may be a potential predictive factor for the risk of developing PBC and act as a protective factor. Further exploration of changes in sphingolipid metabolism and its pathways in PBC could hold significant importance for the development of new therapies for PBC. Docosadienoate (22:2n6) is a C22 fatty acid, belonging to polyunsaturated fatty acids (PUFAs), and it influences the activity of DNA metabolic enzymes such as polymerases (pols) and DNA topoisomerases (topos) [39]. While PUFAs have been implicated in cancer prevention and possess the ability to inhibit topoisomerase activity [40-42] – a mechanism exploited by certain anticancer agents – the role of docosadienoate (22:2n6) in hepatic pathologies remains uncharted. The study by Yonezawa et al. [39] suggests that C22 fatty acids can inhibit the activity of human DNA topoisomerases without affecting the activity of other DNA-related enzymes. Among C22 fatty acids, docosadienoate (22:2n6) is the most potent inhibitor [39], making it a potential anticancer fatty acid among PUFAs. Our study extends this dialogue to the realm of liver disease, calling for dedicated investigations to unravel the potential implications of docosadienoate (22:2n6) in the context of PBC and its pathogenesis.
This study also identified two plasma metabolites, homoarginine and campesterol, which may be associated with an increased risk of PBC. Homoarginine is a non-protein cationic amino acid and shares homology with arginine, with arginine promoting the synthesis of homoarginine [43]. Van der Zwan et al. [44] and the LURIC study [45] found a positive correlation between homoarginine and blood pressure. Furthermore, homoarginine levels are related to glomerular filtration rate, and low plasma homoarginine concentration is a predictor for the progression of kidney disease and major endpoints [46]. While the focus of homoarginine research has been primarily on cardiovascular, cerebrovascular, and kidney diseases, its role in liver health is less understood. Pilz et al. [47] found that homoarginine was negatively correlated with liver function impairment and mortality in outpatient liver cirrhosis patients. Another study [48] identified a significant correlation between homoarginine and plasma alkaline phosphatase activity. However, after adjusting for Child-Pugh stage or MELD score, this correlation weakened. Nevertheless, the details of this relationship, particularly in the context of PBC, demand further investigation. Our study suggests that plasma homoarginine could predict PBC occurrence, which is a significant step toward understanding this amino acid’s role in liver pathophysiology.
Campesterol is one type of plant sterol and serves as a surrogate marker for cholesterol absorption in the intestines [49]. Previous studies have suggested that dietary supplementation with plant sterols can be beneficial, but there are studies indicating potential adverse effects on health. For instance, plant sterols have been associated with inducing endothelial cell dysfunction and inhibiting cell growth [50, 51]. Nikkila et al. [52] reported that PBC patients had elevated plasma campesterol levels. The measurement of plasma cholesterol, cholestanol, cholesterol/campesterol ratio, and campesterol/sitosterol ratio contributed to the staging of liver damage in PBC patients. They also found that, compared to healthy individuals, PBC patients had normal plasma cholesterol levels before liver transplantation, but campesterol levels were 1.6 times higher [53]. Our study suggested that elevated plasma campesterol levels may be a factor increasing the risk of PBC, pointing to dietary intervention as a potential preventive strategy.
In conclusion, our study investigating the causal relationship between plasma metabolites and the risk of PBC has strong theoretical foundations and significant clinical research value. The application of stringent quality control and a variety of MR methods lends credibility and substance to our findings. Moreover, the study’s approach – assessing a wide spectrum of metabolites – adds a layer of complexity and novelty compared to previous studies with narrower scopes.
Nevertheless, our study had some limitations: 1) The GWAS data for plasma metabolites and PBC originated from European populations, which did not encapsulate the full spectrum of global genetic diversity and lacked detailed individual-level data. Consequently, analyses of demographic factors such as gender and age were constrained. 2) There was limited research on the selected plasma metabolites and their relationship with the disease, requiring further exploration. 3) Despite utilizing the largest available GWAS dataset for plasma metabolites, expanding the sample size is necessary for a more accurate assessment of the genetic impact of metabolites on the disease.
Conclusions
In summary, our study harnessed the analytical power of a two-sample Mendelian randomization approach to investigate the causal relationship between an extensive array of 1,400 plasma metabolites and PBC. We identified four plasma metabolites that may have a potential causal relationship with the development of PBC. Sphingosine 1-phosphate and docosadienoate (22:2n6) may reduce the risk of PBC, while homoarginine and campesterol may increase the risk. Furthermore, directional MR analysis did not reveal a causal association between PBC and plasma metabolites.
These findings provide valuable leads that may inform future research and enhance our understanding of the genetic and metabolic underpinnings of this complex autoimmune liver disease.
Disclosures
This work was supported by the Beijing Nova Program (No. Z201100006820051 to XY), and the National Key Research and Development Program (No. 2021YFC0864801, JR).
We used data from publicly available GWAS databases. Studies included in the analysis were approved by relevant institutional review committees, and participants provided informed consent.
The authors declare no conflict of interest.
Supplementary materials are available on the journal’s website.