Introduction
Over the past decade, left atrial appendage occlusion (LAAO) has emerged as an established alternative to oral anticoagulation for patients diagnosed with atrial fibrillation (AF) [1, 2]. The increasing recognition and effectiveness of these therapeutic approaches have prompted rapid advancements in LAAO device technology. Notably, the new cardiology guidelines released by the European Society of Cardiology (ESC) in August 2020, pertaining to AF management, have substantiated the efficacy and validity of LAAO treatments for patients with contraindications to oral anticoagulation [3]. Currently, the atrial fibrillation market is predominantly influenced by endocardial products, extensively employed in clinical practice, with their outcomes corroborated by multicenter studies [4].
Epicardial devices, with the LARIAT device as a notable example, are comparatively less utilized in AF management, despite offering certain advantages over their endocardial counterparts. Epicardial LAAO is recommended for patients intolerant of antiplatelet therapy. Additionally, following epicardial LAAO, the risk of device embolization or thrombus formation is eliminated [5]. However, data pertaining to the long-term outcomes of the epicardial LAAO procedure remain notably scarce.
Aim
The primary objective of this study was to present the extended results (spanning over 9 years of observation) within a cohort of 121 patients with AF who underwent the epicardial LAAO procedure.
Material and methods
All patients received comprehensive information regarding the procedure and provided written informed consent. The study protocol obtained approval from the Polish Ministry of Health and the Ethics Committee at John Paul II Hospital in Krakow, Poland.
This prospective, single-center study encompassed 121 patients referred between July 2010 and May 2013 for left atrial appendage closure (LAAC) using the LARIAT device. Briefly, the procedure involves three primary components: a compliant occlusion balloon, two magnet-tipped guidewires, and a 12-Fr suture delivery device. Employing a percutaneous pericardial approach, a trans-septal puncture is executed. The first endocardial magnet-tipped guidewire is positioned near the apex of the left atrial appendage (LAA). Through percutaneous femoral access, the second endocardial magnet-tipped guidewire is positioned at the LAA’s tip, establishing a secure connection between the guidewires. Subsequently, the LARIAT snare device is advanced over the epicardial guidewire to effect LAA occlusion. Following confirmation of LAA closure via transesophageal echocardiography (TEE) and fluoroscopy, a pre-tied suture is deployed and tightened to ligate the LAA. Notably, no periprocedural colchicine was administered for pericarditis prophylaxis during the procedure.
Follow-up assessments were conducted at 3 and 6 months post-procedurally, each including transesophageal echocardiography. Leaks were defined as the presence of flow from the left atrium to the LAA exceeding 5 mm. Additionally, annual telephone follow-up visits were conducted after the procedure. The physician who performed the procedure was responsible for data collection and reporting of adverse events. Adverse events and mortality were documented during follow-up visits in accordance with the Munich LAA consensus document and the VARC-2 consensus document.
Long-term survival data
Retrospective data were extracted from the KROK (Krajowy Rejestr Operacji Kardiochirurgicznych) (Polish National Registry for Cardiac Surgery Procedures) registry, which is accessible at www.krok.csioz.gov.pl. This registry constitutes an ongoing, nationwide, and multi-institutional database encompassing all-encompassing cardiac surgical interventions in Poland. It systematically gathers in-hospital data concerning patients and outcomes. The registry’s structure has been described in detail in other academic publications.
Regarding long-term survival data, these are directly integrated into the registry from follow-up mortality information acquired from the National Health Fund. This fund operates as a mandatory, comprehensive public health insurance institution on a nationwide scale within Poland. The assimilation of these data into the registry contributes to its comprehensiveness and accuracy.
Post-procedure anticoagulation
Aspirin-based antiplatelet therapy was initially advised for all patients. However, the ultimate therapeutic approach exhibited variability attributed to patient-specific comorbidities, contraindications, and the discretion of the attending physician.
Thromboembolism and bleeding reduction calculation
Adverse events documented during follow-up visits, in accordance with the Munich consensus document, encompassed various categories, notably mortality (cardiovascular, non-cardiovascular, procedural, immediate procedural), thromboembolic events (stroke, transient ischemic attack (TIA), systemic embolism), and bleeding (life-threatening or incapacitating, major, minor).
Similar to our preceding study, the individual annual risk of each patient was meticulously recorded, and subsequently, the mean annual risk for the entire study cohort was computed [6]. The cumulative count of thromboembolic events observed throughout the overall follow-up duration was divided by the cumulative patient-years of follow-up. This quotient was then multiplied by 100 to derive the actual annual rate of thromboembolic events. The assessment of thromboembolism reduction was conducted through the following formula: (estimated percentage – actual percentage event rate) divided by estimated percentage event rate.
The procedural efficacy in averting thromboembolic events (such as stroke, TIA, systemic embolism, and intracardiac thrombus formation) was gauged by contrasting the factual event rate with the rate forecasted by CHA2DS2-VASc scores. The calculation of annual risk encompassed both individual patients and the collective study population.
The assessment of bleeding reduction followed a similar approach. Actual bleeding events were juxtaposed with the projected events based on the HAS-BLED score to evaluate the degree of reduction achieved.
Statistical analysis
Continuous variables underwent assessment for normal distribution using the Shapiro-Wilk test. The data are presented as either mean ± standard deviation or as median (interquartile range; Q1 – 25th percentile and Q3 – 75th percentile), unless otherwise specified. In instances where non-parametric tests were employed, the obtained data were also displayed as mean ± standard deviation to facilitate comparison with other relevant studies. Categorical variables were presented as counts and percentages.
Baseline characteristics across groups were subjected to comparison utilizing the t-test for continuous variables and the χ2 test for categorical variables. Kaplan-Meier analysis was performed to extrapolate survival estimates across time intervals. All statistical analyses were carried out using Statistica 10.0 software (StatSoft, Tulsa, OK, USA). A two-sided p-value of less than 0.05 was regarded as statistically significant.
Results
Baseline patient characteristics are summarized in Table I. The average CHADS2 score was 1.9 ±1.0, the mean CHA2DS2-VAS score was 2.8 ±1.5, and the HAS-BLED score averaged 2.7 ±1.0. Prior to the LAAC procedure, thromboembolic events were documented in 22.3% of cases.
Table I
Patient characteristics
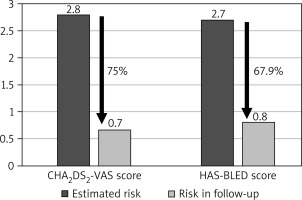
The average duration of follow-up was 74.18 months, with the longest follow-up period extending beyond 8.4 years. Cumulatively, the study population amassed 748 patient-years of follow-up. Throughout the study’s duration, the mean annual rate of thromboembolic events was 0.7%. This encompassed 2 cases of stroke, 1 transient ischemic stroke, and 2 instances of thrombi formation within the left atrium, which subsequently resolved with the administration of unfractionated heparin, yielding no further complications. Notably, this equated to a 75% reduction in the estimated thromboembolic risk. The yearly occurrence rate of major bleeding complications was 0.8%, encompassing three hemorrhagic strokes, two gastrointestinal bleeds, and one urinary tract bleed. The calculated risk of bleeding was effectively reduced by 67.9% (Figure 1).
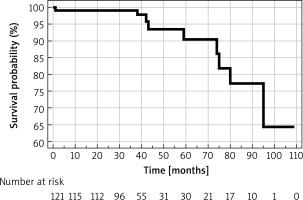
The overall annual mortality rate was 1.2%. The causes of death included cardiovascular disease in 3 cases, with the remaining attributed to lymphoma, pneumonia, sepsis (as a complication of orthopedic surgery), and progression of Alzheimer’s disease. In 2 cases, the immediate cause of death could not be definitively ascertained. Survival estimates, established via Kaplan-Meier analysis for all adverse events, are graphically represented in Figure 2.
Discussion
Non-surgical epicardial LAAO closure has evolved into a standard treatment modality for AF at our center [7]. In this study, we outline the enduring outcomes of the initial patient cohort undergoing epicardial LAAO. Our findings demonstrate robust long-term results, showing substantial reductions in both thromboembolic and bleeding risk. It is noteworthy that the presented results encompass the lengthiest follow-up period documented in the extant literature.
The LAA holds a pivotal role as the primary source of thrombus in AF patients, as confirmed through autopsy, radiological investigations, and molecular analysis. The HEART-CLOT study revealed that the LAA distinctly maintains a localized state, evidenced by the formation of dense fibrin clots [8]. Blood derived from the LAA exhibits diminished fibrin clot permeability and a protracted clot dissolution time when contrasted with peripheral blood and various heart chambers, including the right and left atria and ventricles. The most recent fundamental research reaffirms the clinical rationale behind left atrial appendage closure for prophylaxis against thromboembolic events in patients with atrial fibrillation [9].
Regrettably, the recommendation grade for the LAAO procedure remains at IIb B in the 2020 update of the atrial fibrillation guidelines, primarily due to the paucity of randomized clinical trials and extended observational studies [3]. Currently, the LAAO procedure may be contemplated for stroke prevention in AF patients with contraindications to long-term anticoagulation therapy, such as those with non-reversible intracranial bleeding. It should be highlighted that LAAO interventions are increasingly performed for secondary prevention of ischemic stroke in patients without contraindications to oral anticoagulants, a stance supported by certain local cardiology societies [10]. Analysis of patients with a history of previous thromboembolic events underscores the notable efficacy of reducing secondary strokes of cardiac origin [11].
Over the past decade, two main approaches have emerged for complete LAA closure. An array of systems for LAA closure has arisen in recent years, with many centered on endocardial closure involving the insertion of a metal occluder within the LAA. An alternative strategy involves epicardial closure using the LARIAT system [12]. These approaches differ in terms of access type, postoperative leakage tendencies, and the necessity for postoperative anticoagulation. Following endocardial procedures, most manufacturers advise immediate antiplatelet therapy to deter occlusive thrombus formation. In the case of epicardial occlusion, post-procedure discontinuation of anticoagulants is plausible. Interestingly, some studies suggest that epicardial LAA occlusion leads to reduced secretion of natriuretic hormones, resulting in lowered blood pressure and heart rate – a phenomenon not observed following endocardial occlusion [13, 14]. This could be attributed to necrosis and tissue atrophy subsequent to LAA occlusion.
The efficacy of endocardial systems has been substantiated through numerous clinical trials and registries, often comparing favorably to oral anticoagulant (OAC) therapy [15]. However, experience with epicardial devices is less extensive. The extended follow-up of epicardial LAAO is confined to a limited number of multicenter registries and/or single-center studies. To date, no randomized clinical trials have directly compared these two methodologies. Nevertheless, a retrospective study contrasting 55 matched patients who underwent endocardial and epicardial LAAO exhibited comparable efficacy in reducing thromboembolic and bleeding events during medium-term follow-up, with a slight advantage observed in favor of epicardial LAAO [16].
In this study, we present the longest-ever outcomes of epicardial LAAO within the pioneering patient group that underwent this procedure globally. Our findings include a 75% reduction in the anticipated rate of thromboembolic events and a 67% reduction in the expected rate of bleeding complications. These results align with those obtained from studies involving endocardial devices such as EWOLUTION [17] and the Amplatzer study [18]. Our outcomes also mirror prior discoveries concerning the epicardial LAAO system. Therefore, our findings indicate excellent efficacy in mitigating stroke risk, despite the heightened risk of thrombosis within an aging research population.
Limitations
This study followed a non-randomized, retrospective, observational design conducted within a single center. The primary limitations inherent in assessing the comprehensive efficacy of LAAO include the absence of a control group and the exclusive reliance on calculated stroke or bleeding risk scores for analytical purposes.