Left ventricular (LV) pseudoaneurysms following confined cardiac wall rupture are among mechanical complications of an acute myocardial infarction (MI), particularly if the latter one remained untreated [1]. Currently in the developed countries, early diagnosis of acute coronary syndrome (ACS) combined with reperfusion/revascularization therapies has led to the marked drop in prevalence of this complication [2]. Thus, they are considered as the rare adverse events following MI. Of note, their diagnosis might be difficult, relying mostly on the echocardiography and magnetic resonance imaging (MRI) [3]. However, it is crucial for outcomes of these patients since false aneurysms (FA) have been prone to fatal rupture. Development of LVFA can lead to heart failure, ventricular arrhythmias and thrombolytic events [4].
We present the case of a patient with a large LV pseudoaneurysm that was disclosed incidentally during hospitalization for recurrent acute coronary syndrome.
A 69-year-old male, with no previous medical history, presented with an acute chest pain. He was a heavy smoker, but with no other risk factors for the coronary artery disease (CAD). On admission, the electrocardiogram (ECG) demonstrated sinus rhythm with the right bundle branch block (RBBB) and Q wave in inferior leads (II, III, AVF). The blood pressure was 115/80 mm Hg with the heart rate of 90 beats per minute and the oxygen saturation (SpO2) of 95%. Due to presence of clinical symptoms suggesting ACS combined with high serum troponin levels, the urgent coronary angiography was performed. Three-vessel disease with the subtotal stenosis of the left anterior descending artery (LAD) and circumflex artery (Cx) with total occlusion of the right coronary artery (RCA) was diagnosed.
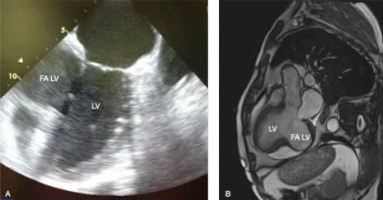
The transthoracic echocardiography revealed the LV aneurysm with maximum dimensions of 49 × 57 mm and an area of a mural thrombus (Figure 1 A), suggesting that it may be a pseudoaneurysm, LV ejection fraction (LVEF) was significantly reduced (approximately 30%). Both LV and left atrium (LA) were enlarged (73 mm and 56 mm, respectively). Additionally, moderate mitral and mild tricuspid regurgitations were observed. The cardiac MRI confirmed the echocardiographic findings in terms of both diagnosis and dimensions (42 × 73 mm). Moreover, there was an area of the mural thrombus up to 35 mm (Figure 1 B).
Figure 1
A – Preoperative echocardiographic study. B – Preoperative magnetic resonance imaging study
LV – left ventricle, FALV – false aneurysm of the left ventricle.
The patient was referred for an urgent surgery and admitted to the Department of Cardiac Surgery and Transplantology for combined aneurysmectomy and coronary artery bypass grafting (CABG).
A standard median sternotomy was performed. After the left internal mammary artery (LIMA) was harvested, the patient was connected to cardiopulmonary bypass (CPB) (Figure 2). With the heart arrested, the inferior wall of the LV was incised and the pseudoaneurysm was resected followed by massive clot evacuation. The edges of the ventricular wall were oversewn using two patches and 2.0 nonabsorbable monofilament suture. The LAD was grafted with LIMA. There was no possibility for RCA grafting due to the pseudoaneurysm location and small diameter of the artery. The patient was successfully weaned from cardiopulmonary bypass (CPB) and after chest closure was transferred to the intensive care unit.
The patient had uneventful recovery and was discharged home on the 7th day after surgery. Post-operative echocardiography showed markedly improved LVEF (about 50%) and only mild mitral regurgitation. Diameters of the LV decreased to 52 mm and that of the LA to 35 mm.
According to the ESC (European Society of Cardiology)/EACTS (European Association for Cardio-Thoracic Surgery) guidelines on myocardial revascularization [5], LV aneurysmectomy during CABG should be considered in patients with NYHA class III/IV, large LV aneurysm, large thrombus formation, or if the aneurysm is the origin of arrhythmias. We are convinced that our patient, although relatively asymptomatic regarding LV aneurysm itself, had clear indications to have the aneurysm excised due to its dimension and thrombus inside.
Additionally, good early outcomes confirmed the appropriate decision to qualify the patient for urgent surgery and removal of the large LVFA. Discharge echocardiographic examination showed not only a significant reduction in LV volume and its geometry restoration but also a significant decrease in a degree of mitral valve regurgitation. Moreover, the surgical approach enabled simultaneous revascularization of LAD territory with LIMA.
Although LVFA is a rare complication of MI, it poses a high risk for the patient if left undetected [6]. Of note, up to a half of all MIs in the general population can be silent (SMI) [7]. They are diagnosed by means of ECG (presence of the pathological Q waves) in patients with seemingly no history of cardiac disease symptoms. Taking into account the incidental disclosure of the LVFA in the present case, we strongly believe that it was a consequence of SMI. Most probably the LV wall rupture had occurred some time before the present coronary incident and was limited by the adherent tissue without an overt symptom. It was followed by the recurrent myocardial ischemia, which resulted in symptom occurrence and hospitalization, which eventually enabled final diagnosis of the LVFA to be established.
The presented case supports the role of very basic and standard studies such as ECG or echocardiography as the useful tools to detect previous MI complications in relatively asymptomatic patients. We are aware that in some cardiological centers access to the catheterization laboratories is even easier than to echocardiography. Other cardiologists decide not to perform the latter study claiming that in ACS patients, time to reach catheterization facilities is crucial to have optimal early and long-term outcomes. However, we strongly believe that echocardiography is necessary in emergency cardiological cases. Moreover, acute cardiac MRI, if the patient is in a stable condition, should be considered. This would enable not only early detection of the pseudoaneurysm, but also its accurate location and guidance for the surgical intervention.