Introduction
Dysfunctional right ventricular outflow tract (RVOT) remains a major clinical challenge after surgical correction of conotruncal abnormalities such as tetralogy of Fallot, pulmonary atresia, and transposition of great arteries or truncus arteriosus [1–4]. Failure of the right ventricle to pulmonary artery (RV-PA) conduit after the Ross procedure is also a well-known scenario [1, 2].
For decades, surgical management was the only option, with redo procedures representing technical challenges and contributing considerably to mortality and morbidity. Introduction of percutaneous pulmonary valve implantation (PPVI) into clinical practice added a valuable alternative option [2, 5, 6]. This approach was pioneered with the Melody valve (Medtronic), followed by the Sapien valve (Edwards Lifesciences). Clinical application of balloon-expandable valves is limited by the size of the RVOT [2, 6].
The recent introduction of self-expanding valves addressees the issue of a dysfunctional dilated RVOT [7–11]. One example is the VenusP-Valve (Venus Medtech, Hangzhou China), designed for implantation into a native or patch augmented RVOT. It consists of a nitinol frame, containing valve leaflets manufactured from porcine pericardium preserved in low-concentration solutions of buffered glutaraldehyde. The maximal commercially available valve diameter is 36 mm. These features make the device a valuable tool in treatment of RVOT not amendable to the previously introduced balloon-expandable valves.
The conventional technique of self-expanding valve implantation requires arterial and double venous access, most commonly bilateral femoral venous access. One of the venous accesses is used for the initial diagnostic catheterization and subsequent introduction of the valve delivery system, and the second venous access is used to perform angiography in the RVOT during valve implantation.
We report a single vein approach for percutaneous self-expanding valve implantation in 2 consecutive patients.
Case 1
A 17-year-old female patient (43 kg) with DiGeorge syndrome and tetralogy of Fallot with absent pulmonary valve underwent RVOT reconstruction with a monocusp patch in infancy. Recently the patient presented with progressive decline of physical exercise capacity. Transthoracic echocardiography showed preserved biventricular function, significantly dilated RV, unobstructed RVOT with massive pulmonary valve regurgitation and dilated pulmonary artery branches.
On chest computed tomography (CT) the diameter of the monocusp valve was 22 mm. The proximal main pulmonary artery (MPA) measured 26 mm, followed by a 17 mm narrowing in the midsection, and widening to 21 mm distally. The coronary arteries were not directly adjacent to the RVOT.
Based on the echocardiography and CT imaging, the patient was initially scheduled for an attempt of a balloon-expanding valve (Melody) implantation. However, during a 34 mm sizing balloon inflation, the RVOT proximally measured 27 mm; the waist on the balloon at the level of the monocusp valve was 25.4 mm and at the MPA near the bifurcation 25 mm. Hence, the RVOT was too compliant and was deemed unsuitable for Melody valve implantation. The patient was scheduled for self-expandable valve implantation.
The procedure was performed 2 months later under general anaesthesia using a single (right femoral) vein approach. After hemodynamic measurements – aorta (Ao)-81/51/65, RV-27/7, right pulmonary artery (RPA)-20/10/13 mm Hg – and initial angiography, a Lunderquist wire (Cook Medical) was placed in the left pulmonary artery (LPA) followed by a 24 Fr DrySeal sheath (Gore) introduced at the level of the MPA. Next, a 28–25 mm VenusP-Valve was precisely implanted in the RVOT using the DrySeal sheath for angiographies. Two angiographies were performed during valve positioning before the beginning of the implantation (Figures 1 A, B), two more during valve deployment, and one after release of the proximal end of the valve before removal of the delivery system (Figures 1 C, D). The valve positioning and deployment required 5 hand contrast injections of 10 ml followed by a rapid push of saline. In total, weight indexed intraprocedural (valve positioning and deployment) contrast utilisation was 1.2 ml/kg.
Figure 1
Biplane two-dimensional angiography images demonstrating a single vein approach for percutaneous self-expanding valve implantation (Patient A). A, B – Positioning of a 28–25 mm VenusP-Valve (Venus MedTech) in the right ventricular outflow tract. Angiography performed through a 24 Fr DrySeal sheath (Gore) used for introduction of the valve delivery system. C, D – Angiography via the sheath after the valve expansion confirmed proper valve position. E, F – Follow-up angiography after valve release and withdrawal of the introduction system demonstrated unobstructed flow to the pulmonary arteries and competent pulmonary valve
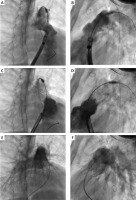
Follow-up angiography through a pigtail catheter in the MPA revealed a correct valve position without regurgitation and unobstructed flow into pulmonary arteries (Figures 1 E, F). Aortography showed a competent aortic valve with proper coronary flow. Final pressure measurement showed the following: LPA 30/21/25, MPA 31/18/24, RV 38/9, Ao 90/55/49 mm Hg.
The post-procedural course was unremarkable. Pre-discharge echocardiography demonstrated a stable position of the VenusP-Valve with a trace of regurgitation. Peak Doppler valve gradient was 15 mm Hg, and the systolic RV pressure estimated from the mild tricuspid regurgitation was 30 mm Hg. Follow-up echocardiography at 6 months showed good function of the valve with minimal central regurgitation and reduction of the RV size.
Case 2
A 12-year-old female patient (37 kg) with tetralogy of Fallot underwent surgical correction in infancy with an autopericardium transannular patch. During a recent outpatient follow-up, the patient reported a slight decline in exercise tolerance. Echocardiography revealed a significantly dilated RV with preserved systolic function, severe pulmonary valve regurgitation, and a wide RVOT and unobstructed pulmonary artery branches. A chest CT showed a dilated proximal RVOT measuring 27 × 34 mm tapering toward the bifurcation to 20 × 19 mm. Coronary arteries were distant from the RVOT. The pyramidal shape of the RVOT raised uncertainty concerning the distensibility of the distal MPA and its ability to accommodate the distal flare of the valve. An inadequate support at the proximal end, even with oversizing of the valve, posed a risk of proximal embolization. Based on the non-invasive imaging, the company experts anticipated a low likelihood of successful Venus-P valve implantation. Balloon testing of the RVOT was suggested.
The procedure was performed under general anaesthesia using a single right femoral vein approach. After hemodynamic measurements (Ao 87/58/70, RV 27/11, RPA 25/10/14), initial angiography was performed. The proximal and mid RVOT measured 31–32.5 mm, and the MPA at the bifurcation was 25 mm. A contrast-filled 34 mm sizing balloon revealed a diameter of 31 mm proximally and expansion to 34 mm in the middle section of the RVOT and 27 mm at the distal MPA. Coronary artery compression and collision with the aortic root were excluded. Using a 24 Fr DrySeal sheath, a 34–25 mm VenusP-Valve was deployed from the RPA. The same technique of imaging as in the first case was used during valve positioning (1 angiography, Figures 2 A, B), implantation (1) and after complete expansion (1, Figures 2 C, D) prior to removal of the delivery system. The number of angiographies was limited to 3 contrast injections of 10 ml followed by a rapid push of saline. In total, weight-indexed intraprocedural contrast utilisation was 1.2 ml/kg.
Follow-up angiography through a pigtail catheter in the MPA confirmed the proper position and function of the valve with unobstructed flow to pulmonary arteries (Figures 2 E, F). The final hemodynamic measurements were as follows: MPA 28/14/20, Ao 88/54/68, RV 34/11 mm Hg.
Pre-discharge echocardiography demonstrated good valve function with trivial central regurgitation and a pressure gradient of 12 mm. Systolic RV pressure derived from mild tricuspid regurgitation was 30 mm Hg. Six months following the procedure, echocardiography demonstrated a stable valve position and good function with trivial central regurgitation.
Discussion
Despite the advances in intraprocedural imaging techniques such as three-dimensional rotational angiography (3DRA), fusion imaging representing a direct overlay of pre-procedure CT or magnetic resonance imaging (MRI) 3D datasets and, most recently, virtual reality modelling (VR), conventional biplane angiography is still the mainstream modality of visualization in the vast majority of catheterization laboratories [10–15]. Recently introduced VR modelling gives better spatial comprehension of intra- and extracardiac anatomy, thus improving preprocedural planning. Still, optimization of conventional angiography imaging, along with prudent contrast usage, plays a major role during such sophisticated and technically demanding procedures as PPVI [15].
In our most recent experience, a single vein approach for self-expanding valve implantation demonstrated adequate intraprocedural visualisation during the valve positioning and deployment. Additionally, this technique allows considerable contrast reduction when compared to the conventional technique with frequent, relatively large volume injections via a separate angiographic catheter introduced through the contralateral femoral vein. Limitation of the contrast volume potentially reduces the incidence of contrast-related side effects, and fewer angiographies, or desirably stored fluoroscopies, lead to overall lower radiation exposure for this young group of patients. The single venous approach resulted in shortening of the procedural time (mean of 135 min for the 2 cases) compared to the conventional (double venous) method used in the remainder of our patients (mean of 157 min). These benefits require further evaluation, as other factors such as the learning curve or additional interventions contribute to the overall radiation exposure, contrast usage and procedural time.
Notably, the outer diameter of the delivery system used for the 28 mm (and 30 mm) valve is 22 Fr, whereas it is 24 Fr for the larger valves. In the first patient, who received the smaller valve, hand contrast injections were easier, with excellent visualization, although the capsule of the delivery system was partially within the DrySeal. In the second patient, contrast injection required more force, and opacification of the RVOT was not as good as in the first example. However, together with additional landmarks created by the pigtail catheter in the aorta indicating origins of the RPA and the LPA (arrows in Figure 2), a few contrast injections were sufficient for precise valve positioning.
Figure 2
Biplane two-dimensional angiography images demonstrating a single vein approach for percutaneous self-expanding valve implantation (Patient B). A, B: Positioning of a 34 × 25 mm VenusP-Valve (Venus MedTech) in the proximal right pulmonary artery (RPA) by means of 24 Fr DrySeal sheath (Gore). The white arrow indicates the origin of the RPA, while the black arrow marks the bifurcation and the origin of the left pulmonary artery. C, D – Release of the valve. Note that the distal part of the flare intentionally protrudes to the origin of the right pulmonary artery (white arrow) and the proximal left pulmonary artery (black arrow). E, F – Final angiogram confirmed the proper position and function of the valve with unobstructed flow to pulmonary arteries
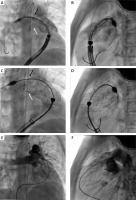
Although there were no technical difficulties related to the single venous approach with several benefits to the patients, the financial cost of a large long sheath, which exceeds the cost of an additional shorth sheath and an angiographic catheter, must be considered.
Single vein technique may be useful in the case of unilateral femoral vein occlusion, which is not an uncommon situation in this group of patients, who often undergo several surgical procedures and multiple heart catheterisations. Additionally, avoidance of bilateral femoral venous access in favour of a single vein approach makes the PPVI procedure overall less invasive and traumatic.








