Introduction
Lung neoplasms are a leading cause of cancer and death worldwide [1]. Lung cancer (LC) has resulted in approximately two million diagnoses and 1.8 million deaths [2], with the age-standardized cumulative lifetime risk of LC diagnosis being 3.8% for males and 1.77% for females [2]. The incidence of LC is increasing globally due to rising industrialization and access to tobacco [3].
In the United States, African American men and Caucasian American women have the highest incidence of LC [4]. A meta-analysis revealed that a positive family history increases LC risk by 1.7 times, and if the history is among first-degree relatives, it can increase the risk by up to 2–4 times even after controlling for smoking history [5].
Genome-wide association studies (GWAS) have confirmed variants in several chromosomal regions related to an elevated heritable LC risk [6–9]. Smoking accounts for over 80% of LC patients in the Western world [2], but exposure to asbestos [10], radon gas [11], air pollution [12], arsenic [13], infections [14, 15], and chronic obstructive pulmonary disease [16] could also be other risk factors for LC.
Chronic inflammation’s durability is a fundamental characteristic of malignant tumors, and inflammation is present in all stages of tumorigenesis [17]. However, the mechanism behind inflammation stimulation in cancer remains largely unknown. Cytokines are small secreted proteins that regulate the immune response by affecting nearly every cell [18] and have a correlation with cancer development [19–22]. Interleukin-6 (IL-6) is one of the most important cytokines, playing a key role in autoimmune diseases, bacterial infections, and metabolic side effects, possessing both pro- and anti-inflammatory properties [23]. IL-6 has been identified as a biomarker due to its primary role in activating and maintaining the inflammatory response [24, 25] and promoting cancer development [20, 26].
Various articles have reported on the serum/plasma levels of IL-6 in LC patients compared to healthy controls (HCs), yielding different results [27–31]. To our knowledge, there have not been any meta-analyses published on this subject in English literature.
Aim
The objective of this study is to conduct a meta-analysis evaluating the serum/plasma levels of IL-6 in LC patients, aiming to provide a more comprehensive and quantitative synthesis of this important biomarker and its potential role in LC diagnosis and treatment.
Material and methods
Study design
The systematic review and meta-analysis were reported using the PRISMA statement [32]. The PECO (Population, Exposure, Comparator, and Outcome) question [33, 34] was formulated as follows: Were blood IL-6 levels different between LC patients and HCs? (human cases with and without LC as the population; LC as the exposure; LC cases compared to HCs as the comparator; and changes in plasma/serum IL-6 levels as the outcome).
Study selection and search strategy
PubMed, Scopus, Web of Science, and Cochrane Library databases were searched to retrieve relevant studies that compared serum/plasma IL-6 levels in LC patients with HCs from each database’s inception until September 11, 2022, without any restrictions. The search strategy used the keywords “IL-6”, “IL6”, or “interleukin-6”, and “lung cancer*”, “lung carcinoma*”, “lung neoplasm*”, “non-small cell lung carcinoma”, “NSCLC”, “small cell lung cancer”, “SCLC”, or “lung adenocarcinoma”, and “blood”, “plasma”, or “serum”. The references/citations of reviews or original articles were checked to ensure that no studies dealing with the subject were missed. Two reviewers conducted an independent check of the retrieved articles (M.S. and S.V.J.), and any disagreement was resolved by a third reviewer (S.Z.).
Eligibility criteria
The inclusion criteria for this study were as follows: 1) case-control studies comparing LC patients to HCs, 2) studies reporting plasma/serum IL-6 levels in both LC patients and HCs, 3) pathological diagnosis of LC, 4) LC patients were over 18 years old and had no other systemic diseases, 5) HCs were over 18 years old and had no LC or systemic disease, and 6) samples of serum/plasma were obtained between 8 am and 10 am. Exclusion criteria included 1) letters to the editor, reviews, book chapters, and conference papers, 2) studies with incomplete data, 3) studies including LC patients under treatment during sampling, 4) measurement of IL-6 levels in samples other than serum/plasma, and 5) studies reporting polymorphisms of mRNA level of IL 6.
Extracted data
Two reviewers (M.S. and S.G.M.) independently extracted the data for the articles included in the meta-analysis, and any disagreement was resolved through discussion.
Quality score
The Newcastle-Ottawa Scale (NOS) [35] was used to assess the quality of each study, with a score of ≥ 7 out of 9 indicating high quality. One reviewer (M.S.) performed the quality assessment for all studies included in the analysis.
Statistical analysis
For statistical analysis, the Review Manager 5.3 software was used to extract effect sizes, including standardized mean difference (SMD), with a 95% confidence interval (CI). Statistical significance was deemed to be present if the two-sided p-value was less than 0.05.
If the Pheterogeneity was less than 0.10 (with I2 more than 50%), significant heterogeneity was present, and the analysis was conducted using a random-effects model [36]. If not, a fixed-effect model was utilized [37]. To explore the heterogeneity of effect estimates in the meta-analysis, a Galbraith (or Radial) plot [38] and L’Abbé plot [39, 40] were employed, with their results and interpretation obtained from NCSS 2021 version 21.0.2 software.
Subgroup analysis, fixed-effect meta-regression analysis (univariate analysis), and sensitivity analyses were also carried out. Bias analyses were performed using Comprehensive Meta-Analysis version 2.0 software, with Egger’s regression test [41] and Begg’s test [42] being used to determine the significant publication bias (p-value (2-sided) of less than 0.10).
To address false-negative or -positive results from meta-analyses [43], trial sequential analysis (TSA) was performed using TSA (version 0.9.5.10 beta) software [44]. The required information size (RIS) was computed with an a risk of 5% and beta risk of 20% for blood IL-6 levels.
The mean difference (MD) was based on empirical assumptions, and if the Z-curve crossed the lines or entered the futility zone, sufficient cases were located in the studies, and the conclusion was reliable. One reviewer (M.S.) performed the analyses, and the other reviewers (S.V.J., S.Z., and S.G.M.) re-checked them.
Results
Study selection
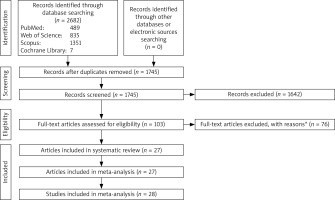
Out of the 2682 records obtained from various databases, only 103 full-text articles qualified based on the eligibility criteria. Upon further examination of the full texts, 78 articles were eliminated for various reasons (as detailed in Figure 1). The remaining 27 articles were chosen for the systematic review, with one article [45] featuring two independent studies. As a result, a total of 28 studies were analyzed in the meta-analysis.
Figure 1
Flowchart of the study selection interleukin-6
*15 studies reported polymorphisms of IL-6. 1 study reported IL-6 mRNA level. 44 studies didn’t include control group or participants were under treatment. 1 study included individuals with several cancers as case group. 1 study measured sputum IL-6. 1 study reported the levels of IL-6 in bronchoalveolar lavage fluid. 1 study reported the levels of IL-6 in breath condensate. 5 studies didn’t include required data for case and/or control groups. 1 study had duplicate data. 2 studies had no full text. 1 study reported fluorescence intensity of IL-6. 1 study reported production of IL-6 by peripheral blood monocytes. 1 study reported geometric means of IL-6. 1 study had irrelevant data.
Characteristics
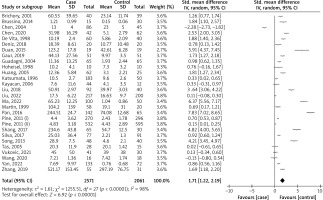
Twenty-eight studies [27–31, 45–66] were published from 1996 to 2022 (Table I). Among all studies, eleven studies were reported in Caucasians, fourteen in Asians, and three in mixed ethnicities. The studies included 2571 cases with LC patients and 2061 HCs. Twenty-four studies reported the levels of IL-6 in serum and four in plasma samples. Stage of LC patients, matched factors between LC cases and HCs, and quality score were other retrieved characteristics of the studies.
Table I
Characteristics of the studies
| First author, publication year | Country/area | Ethnicity | Number of cases | Number of controls | Stage | Blood sample | Matched factors (age and/or sex) | Quality score |
|---|---|---|---|---|---|---|---|---|
| Brichory, 2001 [46] | USA | Mixed | 40 | 39 | I–III | Serum | NR | 6 |
| Brussino, 2014 [47] | Italy | Caucasian | 15 | 30 | I–II | Serum | Age and sex | 9 |
| Chen, 2004 [27] | China | Asian | 86 | 45 | I–IV | Serum | NR | 7 |
| Chen, 2020 [48] | China | Asian | 42 | 62 | NR | Serum | Age and sex | 8 |
| De Vita, 1998 [49] | Italy | Caucasian | 60 | 40 | III | Serum | Age and sex | 9 |
| Deniz, 2018 [50] | Turkey | Caucasian | 20 | 20 | III | Plasma | NR | 7 |
| Duan, 2015 [28] | China | Asian | 19 | 19 | I–III | Serum | Age and sex | 9 |
| Gaur, 2019 [51] | India | Asian | 51 | 51 | I–IV | Serum | Age and sex | 9 |
| Guadagni, 2004 [52] | Italy | Caucasian | 65 | 65 | I–IV | Plasma | Age and sex | 9 |
| Hoheisel, 1998 [53] | Germany | Caucasian | 10 | 10 | NR | Serum | NR | 6 |
| Huang, 2005 [54] | China | Caucasian | 62 | 25 | I–IV | Serum | NR | 7 |
| Katsumata, 1996 [55] | Japan | Asian | 183 | 50 | I–IV | Serum | NR | 7 |
| Kayacan, 2006 [56] | Turkey | Caucasian | 44 | 12 | I–IV | Serum | Age | 8 |
| Liu, 2018 [58] | China | Asian | 92 | 40 | III–IV | Serum | NR | 7 |
| Liu, 2022 [57] | China | Asian | 217 | 200 | I–III | Serum | Age and sex | 9 |
| Ma, 2022 [29] | China | Asian | 100 | 50 | III–IV | Plasma | Age and sex | 9 |
| Martín, 1999 [59] | Spain | Caucasian | 58 | 20 | I–IV | Serum | NR | 7 |
| Pan, 2016 [60] | China | Asian | 142 | 65 | I–IV | Serum | Age and sex | 9 |
| Pine, 2011 [45] | Europe | Caucasian | 270 | 296 | I–IV | Serum | Age and sex | 8 |
| USA | Mixed | 532 | 595 | I–IV | Serum | Age and sex | 8 | |
| Shang, 2017 [61] | China | Asian | 65 | 30 | I–IV | Serum | NR | 7 |
| Silva, 2017 [62] | Brazil | Mixed | 77 | 91 | I–IV | Plasma | Sex | 8 |
| Song, 2013 [63] | China | Asian | 48 | 40 | I–IV | Serum | Age and sex | 9 |
| Tas, 2005 [64] | Turkey | Caucasian | 28 | 15 | III–IV | Serum | NR | 7 |
| Vukoviæ, 2021 [30] | Serbia | Caucasian | 41 | 30 | III–IV | Serum | NR | 7 |
| Wang, 2020 [31] | China | Asian | 16 | 18 | I–IV | Serum | Age and sex | 9 |
| Yan, 2022 [65] | China | Asian | 133 | 72 | I–IV | Serum | Age and sex | 9 |
| Zhang, 2019 [66] | China | Asian | 55 | 31 | NR | Serum | NR | 6 |
Pooled analysis
The pooled SMD was 1.71 (95% CI: 1.22, 2.19; p < 0.00001; I2 = 98%) comparing the blood IL-6 levels in LC patients to HCs (Figure 2). Therefore, the result showed that the blood IL-6 level in LC patients was significantly higher than in HCs.
Subgroup analysis
The subgroup analysis based on sample size, ethnicity, quality score, matched factors, and blood sample is shown in Table II. The results showed that just the quality score could be a confounding factor for the pooled analysis.
Table II
Subgroup analysis
Meta-regression
A meta-regression analysis revealed that publication year, sample size, and quality score were effective factors for the pooled analysis (Table III). With increasing publication year and quality score and decreasing sample size, pooled SMD of blood IL-6 level in LC patients compared to HCs decreased.
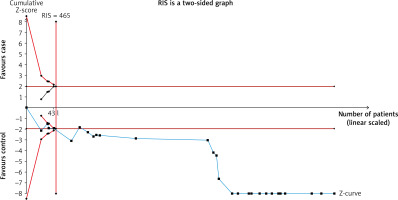
Trial sequential analysis
To report sufficient data for the comparison of blood IL-6 levels in LC patients to HCs, the TSA result was presented (Figure 3). The Z-curve crossed the RIS line and therefore the result confirmed that there were sufficient cases for reporting this comparison.
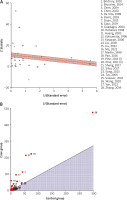
Heterogeneity plots
The radial plot (Figure 4 A) and L’Abbe plot (Figure 4 B) showed that there was possible heterogeneity in the pooled analysis and this heterogeneity may be due to the outliers. The radial plot places studies with the highest weight closest to the Y axis, while studies that fall outside the limits are perceived as heterogeneous and may be considered outliers. On the other hand, for the L’Abbe plot, studies showing heterogeneity are positioned far away from the diagonal line. The study displaying the most heterogeneity is located further away from the limits or the diagonal line.
Sensitivity analyses
The sensitivity analyses including one-study-removed (Figure 5 A) and cumulative (Figure 5 B) analyses showed the stability of the pooled result.
Publication bias
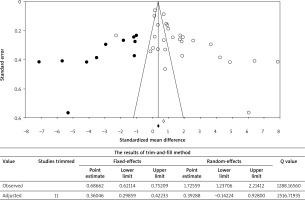
The funnel plot including the missing studies imputed by the trim-and-fill method is shown in Figure 6. The Begg’s and Egger’s tests reported a high publication bias for the comparison of the blood IL-6 levels between LC patients and HCs (p-values: 0.003 and < 0.001, respectively.). Eleven missing studies are filled in the plot. The pooled SMD with pseudo 95% CI was 0.687 (0.621, 0.752) for a fixed-effects model, which, after being adjusted using the trim-fill method, became 0.360 (0.299, 0.422). In addition, the pooled SMD with pseudo 95% CI was 1.725 (1.237, 2.214) for a random-effects model, which, after being adjusted using the trim-fill method, became 0.392 (–0.142, 0.928). The forest plot presented an overall effect size on the plasma/serum levels of IL-6 that seemed to be invalid, as there was a significant publication bias effect based on both fixed-effects and random-effects models. This was due to a considerable difference between the observed and adjusted estimates, although the random-effects model appeared to have a greater effect.
Figure 6
Funnel plot and trim-and-fill method. The open dots show the observed studies, and the closed dots show the missing studies imputed by the trim-and-fill method. The dashed lines that create a triangular area indicate the 95% confidence intervals, and the vertical solid line illustrates the overall effect size
Discussion
According to the main findings of the meta-analysis, the serum/plasma IL-6 level was significantly higher in LC patients as compared to HCs. The analysis revealed that the year of publication and quality score had a positive correlation while sample size had a negative correlation with the pooled SMD for comparison of serum/plasma IL-6 levels. Subgroup analysis indicated that the quality score could be a confounding factor for the pooled analysis.
Out of all the articles included in the meta-analysis [27–31, 45–66], only one study [27] reported a significantly lower level of IL-6, while 20 studies [28, 29, 39, 45–52, 54, 55, 58–62, 65, 66] found a higher level of IL-6 in LC cases than in HCs. The remaining studies [30, 31, 53, 56, 57, 64] did not find any significant difference between the two groups. Some studies [57, 58, 67] showed that serum IL-6 was linked to anxiety and depression in LC cases. Martin et al. [59] concluded that serum IL-6 levels were higher in LC patients who experienced weight loss of more than 10% as compared to those who had less than 10% weight loss. The varying results observed in the original articles can be attributed to differences in sample sizes, quality scores, and even different methods used by the studies. As per the meta-analysis, it is recommended to account for environmental factors such as smoking, psychological conditions, and nutritional status; however, this meta-analysis could not consider these factors due to insufficient data.
IL-6 is an inflammatory biomarker that plays a critical role in immune responses and inflammation [68]. Chronic inflammation and cytokine storm are uncontrolled forms of inflammation [68], and IL-6 is produced by senescent cells and is involved in aging-induced inflammation and age-related pathologies and cancer [69]. Several recent meta-analyses have reported higher levels of serum/plasma IL-6 in patients with various diseases, including obstructive sleep apnea syndrome [70], kidney disease [71], hepatocellular carcinoma [72], COVID-19 [73, 74], and colorectal cancer [75], compared to controls.
Inflammation has been linked to cancer development and progression and can advance all stages of tumorigenesis [76]. Chronic inflammation, infection, or autoimmunity in the same tissue or organ site causes about 15% to 20% of all cancers [77, 78]. The inflammation that leads to cancer is triggered and present well in advance of the actual tumor formation in such instances [79].
The development of LC is associated with various factors that cause inflammation in the lungs, such as smoking, tuberculosis, occupational exposure to dust, and idiopathic pulmonary fibrosis [80, 81]. Similar to the present meta-analysis, higher serum/plasma IL-6 levels in LC cases could be attributed to inflammatory processes in the lungs. Therefore, it is crucial to consider the role of patient characteristics, such as LC stage and subtype, since the numbers of patients differ in terms of these factors in the studies. One study stated that serum levels of IL-6 differ significantly between LC subtypes, with large cell carcinoma showing higher levels than adenocarcinoma. Another study [49] found that serum levels of IL-6 were significantly higher in LC cases with stage IV than in stage III, and there was also a significant difference between stage III/IV and stage I/II [51]. Additionally, Shang et al. [61] observed that the serum level of IL-6 in LC patients with lymph node metastasis was significantly higher than in patients without metastasis.
The present meta-analysis contributes to our understanding of the role of IL-6 in LC development and progression, indicating that high serum/plasma levels of IL-6 could be a potential biomarker for this disease. From a clinical perspective, this may have significant implications in early detection and diagnosis of LC, especially for high-risk groups such as smokers or those with occupational exposure to toxins. Additionally, identifying IL-6 as a possible mediator of inflammation-induced carcinogenesis may lead to new therapeutic targets for the prevention and treatment of LC.
However, it is essential to note that the meta-analysis has several limitations, including high heterogeneity across studies, publication bias, and insufficient data regarding the impact of psychological and environmental factors on IL-6 levels. Moreover, variability in the methods used for measuring IL-6 levels across studies could potentially affect the results. Additionally, there could be other unmeasured confounding factors, such as other inflammatory markers or comorbidities, which may influence the association between IL-6 and LC.
Conclusions
According to the results of this meta-analysis, patients with lung cancer (LC) have notably higher levels of serum/plasma IL-6 in comparison to HCs. This discovery has significant clinical implications as it suggests that IL-6 may be a useful biomarker for the detection and monitoring of LC. Moreover, targeting the IL-6 pathway could be a promising strategy for managing inflammation in patients with lung cancer.
However, additional research is necessary to comprehensively comprehend the role of IL-6 in LC and its potential as a biomarker or therapeutic target. Future investigations could explore the correlation between IL-6 levels and LC prognosis, as well as the effectiveness of IL-6-targeted therapies in the treatment of LC. Overall, this study offers important insights into the possible involvement of IL-6 in LC and emphasizes the need for further research in various areas.