Summary
We present long-term outcomes in CIRCULATE-AMI Pilot Study Cohort that evaluated safety and feasibility of transcoronary administration, in large-infarct subacute phase, of 30 × 106 standardized Wharton jelly mesenchymal stem cells as an off-the-shelf ‘unlimited’ therapeutic agent to stimulate myocardial repair and regeneration. Evolution of left ventricular volumes and ejection fraction throughout 3 years showed a lasting improvement in myocardial contractility and minimal adverse remodeling; an effect consistent in all diagnostic methods used (SPECT, echocardiography, cMRI). No safety concerns occurred throughout 3 years. Long-term follow up in CIRCULATE-AMI Pilot Study Cohort suggests that Wharton jelly multipotent stem cells transcoronary application ≈ 5–7 days after large acute coronary intervention in humans may be associated with inhibition of left ventricle adverse remodeling and an improvement in left ventricular ejection fraction that is sustained at 3 years. This hypothesis is being tested in a double-blind randomized controlled study.
Introduction
Ischemic heart disease is a leading cause of morbidity and mortality in developed countries [1, 2]. Coronary heart disease is the leading cause (41.3%) of deaths attributable to cardiovascular disease (CVD) in western societies, followed by stroke (17.2%), high blood pressure (11.7%), heart failure (9.9%), diseases of the arteries (2.8%), and other CVDs (17.3%) [2]. Each year CVD not only causes nearly 4 million deaths in Europe but also is an important contributor to chronic disability [3]. Despite unquestionable progress in pharmaco- and device therapies, there is a need to develop novel therapeutic approaches to address the growing burden of cardiovascular disease [4, 5].
Myocardial infarction (MI) is defined as the death of cardiac muscle resulting from prolonged ischemia associated with coronary arthrosclerosis, acute plaque rupture or erosion and imposed thrombosis. In the last decades the death counts in acute coronary syndromes have been reduced by clot-busting drugs and developing new therapeutic and logistic strategies in acute percutaneous coronary interventions and arterial stents. This, however, has led to increasing numbers of patients struggling with the chronic consequences of CVD [5].
Regenerative medicine and cell-based therapies opened a new strategic field to reduce the loss and facilitate rebuilding the damaged tissue in acute coronary syndromes by limiting myocardial remodeling and fibrosis – and thus attenuating progression of acute ischemic injury to chronic ischemic heart failure. Mesenchymal stem cells (MSCs) were isolated from bone marrow in 1970 [6]. These fibroblast-like, nonhematopoietic, plastic adherent cells are able to proliferate in vitro and may be retransplanted in vivo [6–8]. The International Society of Cellular Therapy defines MSCs as self-renewing, multipotent cells with capability of plurilineage or multilineage differentiation potential expressing CD73 and CD90 but not CD45, CD34, CD14, CD11b, CD79a, CD19, or HLADR surface markers [9]. Several different MSC types are being evaluated in preclinical and clinical studies [10]. MSCs are present in bone marrow [6], placenta [11], adipose tissue [12], lung [13], umbilical cord [14], synovial membrane [15], dental pulp [16] and other organs [17]. A major challenge in regenerative medicine today is selection of the best sort of cells, standardization of preclinical experiments and clinical trials, optimizing cell delivery methods, defining an optimal dose and timing of cell transfer, obtaining sufficient cell quantities and improving their delivery and uptake to the target zone [10].
Wharton’s jelly mesenchymal stem cells (WJMSCs, umbilical cord mesenchymal stem cells) are naturally present in the umbilical cord, are free of ethical concerns and can be easily harvested and isolated in abundance [18], making them ideally suited for use as an “unlimited” off-the-shelf source of regenerative/reparative cells [14, 19, 20].
Aim
The aim of the present study was to evaluate the feasibility, safety and impact on left ventricular (LV) remodeling following WJMSC transplantation in CIRCULATE-AMI PSC, as a novel myocardial regeneration strategy using WJMSCs as an off-the-shelf ‘unlimited’ therapeutic agent.
Material and methods
Ten consecutive patients (age 32–65 years) with their first large MI defined as sustained left ventricular ejection fraction (LVEF) reduction to ≤ 45% and peak creatine kinase-MB (CK-MB) level exceeding 100 U/l (upper limit of normal (ULN) < 24 U/l) were enrolled in CIRCULATE-AMI PCS [19]. In the case of ≥ 1 ischemic coronary lesion in 1-vessel or 2-vessel CAD, complete coronary revascularization was required prior to cell transfer. Patients with three-vessel disease were not recruited. The study was compliant with the Declaration of Helsinki, and it was approved by the local Ethics Committee. All subjects provided informed written consent to participate [19].
Study group characteristics at baseline were the following: peak hs-troponin T 17.3 ±9.1 ng/ml, peak CK-MB 533 ±89 U/l, left ventricle ejection fraction (LVEF) by gadolinium-enhanced cardiac magnetic resonance imaging (cMRI) 40.3 ±2.7%, and infarct size (IS) 20.1 ±2.8% (Table I).
Table I
Pilot study cohort characteristics
| Variable | Mean ± SEM (min.–max.) or n (%) |
|---|---|
| Age [years] | 55.6 ±3 (32–65) |
| Women | 5 (50) |
| Diabetes | 3 (30) |
| Prior myocardial infarction (MI) | 0 (0) |
| IRA = LAD/Cx/RCA | 6/3/1 (60/30/10) |
| IIb/IIIa rec. inhibitor (abciximab) | 4 (40) |
| IRA DES/BVS | 9/1 (90/10) |
| IRA TIMI-3/TIMI-2* | 8/2 (80/20) |
| Peak hs-troponin T [ng/ml] | 17.3 ±9.1 (5.2–-102.1) |
| Peak CK-MB [U/l] | 533 ±89 (148–965) |
Echocardiography, cMRI, and ECG-gated single photon emission computed tomography (SPECT) were performed within 24–48 h prior to cell transfer [19], consistent with our earlier protocols [21, 22].
In CIRCULATE-AMI PSC, the clinical and imaging [23] follow-up was throughout 3 years. SPECT was performed at baseline and 1 year (in the first 5 patients also at 6 months). Echo and cMRI were performed at baseline, at 6 months (in the first 5 patients) and then at 1 year and 3 years in all study subjects.
Single photon emission computed tomography
SPECT was performed using a Siemens Symbia T16 SPECT/CT machine. End-diastolic and end-systolic volume, stroke volume (SV), and ejection fraction of the left ventricle (LV) were calculated using quantitative gated SPECT (99mTc-sestamibi) [24].
Cardiac magnetic resonance imaging
cMRI (3T Siemens Magnetom Skyra, 1.5T Siemens Magnetom Avanto) was performed using standard protocols as described previously [22]. Typical parameters included left ventricular end-diastolic and end-systolic volume (LVEDV, LVESV), SV, ejection fraction of the left ventricle (LVEF) and infarct size (IS). Infarct mass and transmural extent of infarction were quantified by contrast-enhanced imaging [22].
Transthoracic echocardiography
Transthoracic echocardiography (TTE; Philips Epiq 7C) involved 2D and M-mode images using standard views: parasternal long axis, parasternal short axis and apical 4 chamber, apical 2 chamber and apical 3 chamber views. Typical parameters included LVEDV and LVESV, and LVEF and SV [25, 26].
Statistical analysis
Data are presented as mean and standard error of the mean (SEM) or as median and interquartile range (continuous variables), or as counts and percentages (categorical variables). The normality of distribution was checked using the Shapiro-Wilk test. Homogeneity of variance was tested using Cochran’s test. Repeated measures ANOVA or Friedman’s test for repeated measures was used for repeated parametric measurements as appropriate. Categorical variables were compared by the χ2 test and Fisher’s exact test. The significance level was set at p < 0.05. Statistical analyses were performed with Statistica version 12 (StatSoft Inc, Tulsa, OK).
Results
Clinical characteristics of the CIRCULATE-AMI pilot study cohort are shown in Table I, indicating recruitment of a typical group of consecutive patients with a large, first AMI. Interventional and pharmacologic treatment was standard and was consistent with the European Society of Cardiology (ESC) guidelines.
At ~5–7 days after AMI, 30 × 106 standardized WJMSCs were administered per protocol via the IRA using a cell delivery-dedicated, coronary-non-occlusive catheter [27].
Myocardial imaging with echocardiography, SPECT and cMRI, as well as WJMSC delivery, were uncomplicated. WJMSC transcoronary administration had no effect on epicardial flow or myocardial perfusion. Specifically, the epicardial flow was TIMI-3 in all subjects before and after cell transfer, and corrected TIMI frame count (cTFC) was 45 ±8 prior to cell transfer and 44 ±9 after cell transfer (p = 0.51). No patient showed hs-troponin elevation within 12 and 24 h after cell delivery. 24 h ECG monitoring was performed in every patient following cell transfer; this did not reveal any significant arrhythmias. The single adverse event that was considered potentially related to WJMSC administration was a transient temperature rise to 38.9°C in 1 patient 2 days after cell transfer – in absence of any symptoms or signs of infection. This was responsive to typical paracetamol treatment, resolved rapidly and was without sequelae.
Within 3 years of follow-up 1 patient died from a new, non-index territory AMI. There were no other major adverse cardiovascular and cerebrovascular events (MACCE) and no adverse events that might be related to WJMSC treatment.
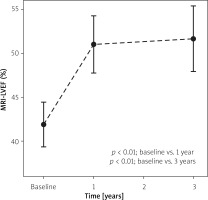
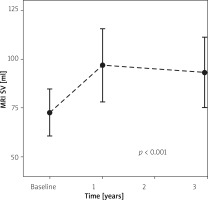
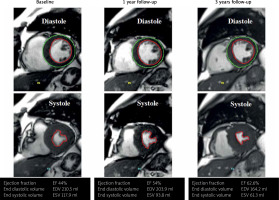
cMRI, SPECT, and echo were all consistent in their demonstration of a significant increase in LVEF between the point of WJMSC administration and 12 months (41.9 ±2.6% vs. 51.0 ±3.3%, 36.0 ±3.9% vs. 44.9 ±5.0%, and 38.4 ±2.5% vs. 48.0 ±2.1% respectively, p < 0.01 for all). There were no significant differences between 6 and 12 months of follow up. An effect that was sustained at 3 years (3 y FU LVEF cMRI 52.19 ±3.96%, echo 50.2 ±2.29%, 1 year vs. 3 years LVEF cMRI and echo p > 0.05). For evolution of SV and LVEF by cMRI see Figures 1 and 2. For a raw-data example of left ventricular volumes and LVEF by cMRI see Figure 3.
Throughout 3 years there were no significant changes in LVEDV by the imaging methods used: SPECT 157.1 ±27.1 ml vs. 174 ±24 ml (baseline vs. 1 year); cMRI 171.6 ±17.7 ml vs. 188.2 ±23.4 ml vs. 181.9 ±17.6 ml (baseline vs. 1 year vs. 3 years), and echo 137 ±13.3 ml vs. 135 ±2.9 ml vs. 148 ±21.8 ml (baseline vs. 1 year vs. 3 years), p > 0.05 for all. However, there was a significant increase in stroke volume measured by SPECT at baseline vs. 1 year (53.3 ±7.6 ml vs. 68.2 ±5.2 ml); cMRI at baseline vs. 1 year vs. 3 years (72.7 ±5.2 ml vs. 96.9 ±8.1 ml vs. 93.2 ±7.8 ml) and echo at baseline vs. 1 year vs. 3 years (64.3 ±2.9 ml vs. 68.8 ±4.1 ml vs. 81.9 ±7.1 ml), p < 0.05 for all.
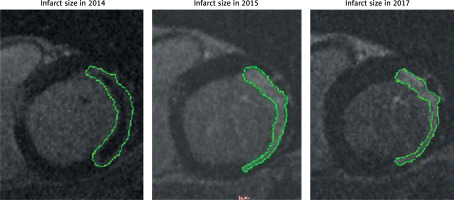
cMRI infarct size was reduced from 33.2 ±7.6 g (baseline) to 25.5 ±6.4 g (1 year) and 23.1 ±5.6 g (3 years); p = 0.03. Figure 4 shows typical evolution of cMRI infarct imaging throughout 3 years.
Discussion
We present long-term outcomes of the CIRCULATE-Acute Myocardial Infarction trial (NCT03404063) Pilot Study Cohort of 10 patients – a first-in-man (FIM) investigation of safety and feasibility study of standardized WJMSC preparation (30 × 106 cells) transcoronary administration through the infarct-related artery at ~5–7 days after AMI. Evolution of left ventricular volumes (LVEDD, LVESD, SV) and ejection fraction throughout 3 years was consistent according to cMRI, SPECT and echocardiographic imaging, all of which indicated a lasting improvement in myocardial contractility and (a degree of) prevention of adverse remodeling (Figures 1–3).
The progress in myocardial revascularization tools and strategies and in pharmacology over the past three decades resulted in a decrease of 1-year mortality in the acute phase of MI from ~30% in the early 1980s to less than ~3% at present [4], reaching a plateau [28]. Today, patients live longer after myocardial infarction, but the prevalence of ischemic heart failure has greatly increased – and it is projected to increase further, presenting a very significant challenge to health care systems [4, 5].
As a result of AMI the heart muscle loses a part of the functional myocardium and undergoes an adaptive response in order to sustain the cardiac output [29]. Remodeling of the left ventricle in response to the increase in wall stress caused by cardiomyocyte loss and distension in the infarct area [30] is the main cause of progressive left ventricular dysfunction and chronic heart failure [2, 31]. The physiology of remodeling includes infarct expansion, chamber dilatation, ventricular hypertrophy, scar formation, neurohormonal responses, cytokine activation, and oxidative stress [31]. The infarct border zone, a primary area of progenitor cell accumulation [22], is a particularly relevant player in left ventricular adverse remodeling [22]. Left ventricle adverse remodeling is usually defined as an at least 15–20% increase in LV end-diastolic volume (LVEDV) [32, 33] and it is associated with significantly poorer clinical outcomes after MI [33, 34]. Both increase in LVEDV (a measure of LV dilatation) and fall of LVEF are part of post-MI left ventricular adverse remodeling, and they are powerful predictors of adverse cardiovascular events after MI [35–40]. Data from the present study suggest that even in patients with large infarcts (such as those in the CIRCULATE-AMI PSC) this degree of pathologic increase in LVEDV can be prevented with WJMSCs delivered through the IRA in the subacute phase of myocardial infarction (example in Figure 3). Indeed, our study demonstrates a significant and sustained improvement in LVEF throughout 3 years, and absence of any significant changes in left ventricular end-diastolic volume by all diagnostic methods used (echocardiography, SPECT, cMRI), in patients who received transcoronary administration of WJMSCs to the infarct zone in the sub-acute phase of MI. In our study, a significant increase in LVEF was paired with increase in stroke volume measured between WJMSC administration and 1 year that was sustained after 3 years in all diagnostic methods.
Left ventricle dilation is associated with an increase of cardiomyocyte size resulting in an increase of left ventricle mass [29]. Our study shows a significant decrease of median left ventricular mass between WJMSC administration and 1 year that is sustained at 3 years, which might result from inhibition of LV hypertrophy.
Another parameter of poorer outcomes after MI is the infarct size. A recent meta-analysis showed that infarct size measured by CMR or 99mTc SPECT within 1 month after the primary percutaneous coronary intervention is strongly associated with all-cause mortality and hospitalization for heart failure within 1 year [41]. In our patient cohort, there was a significant reduction in infarct size by cMRI during 3 years of observation.
It needs to be emphasized that apart from the ischemic component of LVEF reduction in AMI, there is also a significant role played by neurogenic injury that shows a ‘spontaneous’ recovery [26]; thus, only a randomized, double-blind, appropriately powered comparison can provide a reliable measure of the WJMSC-dependent effect.
Our present findings provide the basis for a larger-scale, randomized evaluation of WJMSC use to improve LV function after a large myocardial infarction. CIRCULATE-AMI is a cardiac magnetic resonance imaging (cMRI) infarct size-reduction-powered double-blind randomized controlled trial (RCT) that enrolled consecutive patients with their first, large AMI (cMRI-LVEF ≤ 45%) with successful infarct-related artery (IRA) primary percutaneous coronary intervention reperfusion to transcoronary administration of Wharton’s jelly multipotent stem cells (WJMSCs) vs. placebo (2 : 1).
This work has limitations that are typical for a FIM study that (due to the sample size and lack of a parallel comparator) can evaluate only feasibility and safety of a novel treatment approach. The present research was designed to be a pilot study as a potential basis for a randomized control trial to evaluate efficacy of WJMSCs in inhibition LV remodeling and inhibition of infarct-dependent reduction in LV contractility.
Conclusions
Long-term follow-up data in CIRCULATE-AMI PSC suggest that WJMSC transcoronary application in a recent large AMI in humans may be associated with inhibition of LV adverse remodeling and an improvement in LVEF that is sustained at 3 years. Although lack of a control group results in a lack of feasibility for any direct comparisons, our present results – taken in relation to the literature data on the degree of LV adverse remodeling with large infarcts [32, 33, 39] – suggest a potential benefit of WJMSC delivery via the IRA in the subacute phase of AMI in terms of reduction of LV adverse remodeling and improvement in contractility. This is consistent with experimental data demonstrating improvement in myocardial contractility with WJMSC administration in small-animal and large-animal models of AMI [42–45].
Our appropriately powered, multicenter, randomized double-blind controlled clinical trial (NCT03404063) will verify the impact of WJMSCs’ transcoronary delivery in subacute AMI on LV remodeling.