Introduction
The most common types of adrenal tumours are benign cortical adenomas [1], although the adrenal gland is one of the most prevalent sites for metastases from various malignancies and metastases are the most frequent malignant tumours of the adrenal gland. The existence of adrenal metastases in patients with a history of cancer varies in different series from 10 to 27% [2, 3]. Usually, they are a part of disseminated disease while isolated adrenal metastases occur only in less than one percent of patients [4].
Surveillance protocols in treated cancer patients have increased the identification of incidental adrenal lesions, of which a high proportion are metastases. More reliable and sensitive methods of diagnostic imaging lead to earlier detection of adrenal metastases.
Selection of patients with adrenal metastases for curative treatment remains a big challenge.
Incidental adrenal masses are detected in approximately 4–5% of patients who undergo CT examinations. The occurrence of adrenal incidentalomas increases with age: from less than 0.5% of adrenal nodules revealed in patients in their 20s compared with up to 7% in patients older than 70 years [5–8].
The majority of these tumours are benign nonfunctional lesions. On the other hand, the frequency of adrenal metastases is high. Opposite to these data, the number of adrenalectomies performed to treat metastases is low, from less than one performed in a surgical centre per year [9] up to less than six a year [10, 11]. It is one of the reasons that makes it difficult to establish criteria to define which patients will benefit from adrenalectomy in terms of cure or extended survival. Published data are heterogeneous, differing in frequency of occurrence of tumours that metastasize to the adrenal glands.
Benefits of adrenalectomy for metastases are still unclear but several retrospective studies have identified highly selected groups of patients whose survival seems to be prolonged by surgery [9, 12, 13].
We have assessed the results of metastatic adrenalectomy in our institution to identify prognostic factors for survival.
Material and methods
This is a single-centre retrospective analysis of patients with adrenal metastases undergoing adrenalectomy in our hospital in which the final histopathologic report showed explicit evidence of adrenal metastatic disease. To identify patients with such diagnosis medical records were reviewed. Pre-operative clinical, radiological and biochemical data and detailed operation and histology reports were retrieved from patients’ medical records, either in paper form or by electronic means. Hence, radiology and pathology reports were intentionally not re-evaluated, implying that the data were purely observational.
Over the period from January 2004 to December 2017, 430 patients with various adrenal tumours were referred to our centre for surgical treatment. In this group, 39 (9.1%) patients with metastases to the adrenal gland were identified. Patients with a direct extension of primary tumour or renal cell carcinoma (RCC) with ipsilateral synchronous metastases were not included in the study as well as patients with suspicion of generalized disease. All other patients with isolated adrenal tumours suspected for malignancy were referred for surgery.
Metastases were defined as synchronous if detected within 6 months after primary surgery and those more than 6 months defined as metachronous. Disease-free interval (DFI) was the time between primary treatment and adrenalectomy.
The decision regarding operative treatment was based on diagnostic imaging. The risk of malignancy was determined by several factors including tumour size, radiographic features such as irregular margins, high density, slow washout of contrast medium, presence of necrosis, area of haemorrhage and calcifications. According to current guidelines, adrenal biopsies were not performed.
All patients were operated on under general anaesthesia with venous thromboembolism prophylaxis by low molecular weight heparins and appropriate antibiotic prophylaxis using a second-generation cephalosporin (cefuroxime) according to the hospital protocol.
For patients with large tumours or essential comorbidities, cross-matched blood was reserved. All patients were operated on by open surgery and the typical surgical approach was transabdominal lateral flank incision. In the presented period laparoscopic adrenalectomy was not used in patients with tumours suspected for malignancy.
21 left, 14 right and 4 bilateral, classical, open adrenalectomies were performed. There were no cases of mortality or major complications associated with surgery. The average time of hospital stay was 7 days (range 4–30). One prolonged hospitalization – 30 days – was related to wound pain requiring the use of opioids. The follow-up period was from 3 to 81 months.
Because most of the patients were referred for surgery from different oncological centres, systemic treatment was performed according to up-to-day protocols outside our centre and its analysis is not a part of this study.
Overall survival (OS) and disease-free survival (DFS) were estimated by the Kaplan-Meier method and differences in survival were compared by the log-rank test. Uni- and multivariable predictors of overall mortality were estimated by Cox regression analysis. A p value of < 0.05 was considered to be significant (Statistica ver. 13.1) (StatSoft, Poland).
Results
Thirty-nine patients with adrenal metastasis (17 female, 22 male), mean age 64.8 years (range 49–79 years), were treated by adrenalectomy in our centre during the study period. The diagnoses were: non-small cell lung cancer (NSCLC) – 15 cases, RCC – 14 cases, colorectal cancer – 6 cases and others (melanoma – 2, ovarian – 1, urothelial cancer – 1) (Table 1).
Table 1
Characteristics of tumours and patients
The mean DFI between primary treatment and adrenal surgery for all patients was 42 months and range 1–179 (Table 2).
Table 2
Interval between primary treatment and adrenal surgery – group characteristics
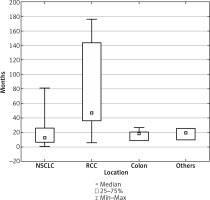
Time from primary treatment to adrenalectomy differed depending on the primary tumour and was the longest for RCC, and this difference was statistically significant (p = 0.000362) (Fig. 1).
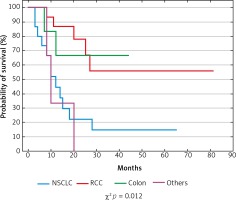
In the presented series median OS (95% CI) was 18 months (mean 22 months) (Fig. 2), and the 2-year actual OS rate was 43.6% (17/39 pts).
Median DFS was 18 months (mean 20.7 months) and varied from 6 (others group) to 25.5 months (colorectal cancer group) although multivariate analysis did not show any independent significant factors for DFS (p = 0.089).
In the univariate analysis, a significant influence on OS was demonstrated for primary tumour type. Significantly better OS was observed for patients with colorectal cancer metastases (median 29.5 months) compared to NSCLC (median 10 months) and others (median 10 months) (p = 0.0119). In NSCLC patients no difference in median OS was found between the synchronic (median – 20, mean – 17.7 months) and metachronous (median – 20, mean – 22.4 months) group (p = 0.787). For RCC median OS was 24 months. None of the patients with melanoma, ovarian or urothelial cancer lived longer than 20 months. There was no statistically significant difference in survival depending on sex, age and metastatic tumour size. However, in the group of patients who died, the size of metastasis (mean 76.5 mm) was significantly bigger than in the group of survivors (mean 52.5 mm) (p = 0.026).
Discussion
Resections of metastases to the adrenals started in the mid-1970s [14]. Then, after a study which showed significantly better survival in patients after resection of adrenal metastases from NSCLC and for those who were treated conservatively, it became an accepted treatment [15]. Later, many publications demonstrated good results of surgical treatment for patients with isolated metastases of different types of cancers in selected patients [16, 17].
There are few prospective data and a lack of randomized evidence to confirm that local therapy to the adrenal metastases alters the natural course of disease. Treatment of patients with adrenal metastases varies among institutions and is strongly dependent on the clinic’s discretion.
In our consecutive 14-year series, we analysed 39 patients treated with adrenalectomy for adrenal metastases of solid tumours. The number of patients is similar to previously reported series [18, 19]. It is worth noting that even in multicentre studies the number of analysed patients rarely exceeds several dozen cases [9, 20, 21].
In the presented group, the predominant cancers which metastasised to adrenals were NSCLC and RCC, which together corresponds to over 74% of metastases. On pooling data, the three most common primary histologies were lung cancer (small and non-small), RCC and melanoma, representing 32%, 22% and 15% of patients respectively [22]. In another large series, most frequent primary tumours were lung (46.6%), colorectal (13.5%), renal (11.7%), breast (3.5%) and melanoma (3.5%) [23]. The relative prevalence of each primary cancer varies according to the source of data and the geographic region. Data from Asia indicated a high prevalence of stomach (14%), oesophagus (12%) and liver/bile duct cancers (10%) with a paucity of breast cancer and melanoma [4], but even in some European series liver and bile duct cancer may be the most frequent (29%) primary tumour metastasizing to adrenals [24].
Mean DFI in the present series was 42 months with the longest interval for RCC (176 months), and these findings were very similar to previously published findings – 39 and 156 months respectively [25]. These findings are worth taking into account in follow-up protocols.
In the present study, the median OS was 18 months and was superior to some studies with median survival of 14 months [26] and similar to other series with 23 months [18]. On the other hand, there are only a few studies with considerably better median survival of 29–32 months [9, 20].
Actual 2-year survival in the present material was 43.6%. This is in line with other investigators, who report a 2-year survival rate of 31–40% [24, 26]; however, there are studies with better survival reaching 61% at 2 years [9].
In patients with adrenal metastases, tumour type was previously suggested to be a prognostic factor for better survival [27]. In the present series tumour type was a significant prognostic factor with the most favourable outcome for patients with colorectal cancer, opposite to the shortest survival observed in patients with NSCLC. These observations are similar to previous reports [18].
Primary renal cancer seems to have a good prognosis in comparison to other primary malignancies. In 41 patients, median OS was 14 months and 5-year survival was observed in 21%, while for patients with primary renal cancer the 5-year survival rate was 50% [24]. Significant differences in survival with regard to tumour type were seen in another report, with longer survival for patients with colorectal carcinoma or RCC and shorter for those with NSCLC or melanoma [18].
Findings of other studies are ambiguous regarding factors which determine survival after adrenalectomy for metastases. In a group of 90 patients with dominant primary melanoma and lung metastases and actual 5-year survival of 26.2%, there was no statistically significant difference in OS for those who underwent adrenalectomy for metastases from lung cancer compared with those with melanoma or other tumours [28].
Not only renal primary but also a tumour size smaller than 5 cm in multivariate analysis were independently associated with longer survival in a series of 65 consecutive patients submitted to adrenalectomy for metastatic disease [25].
Analysis of the present series did not reveal that the median duration of survival depends on tumour size, and this finding is concordant with other reports [17, 29]. Opposite to this, there are studies which demonstrate that adrenal metastases with large diameter (> 45 mm) are associated with a worse prognosis [18]. There are also series in which analysis of the entire group did not reveal that the median duration of survival depended on tumour size except in the colorectal cancer group where a significant difference in favour of the smaller tumours (< 60 mm) was found [10].
Since the first report of laparoscopic adrenalectomy [30], it has become the gold standard of treatment for benign adrenal masses [31] and is gaining acceptance in treating metastatic adrenal lesions [10, 21, 32, 33].
All patients in the present material were operated on by open surgery. Laparoscopic adrenalectomy in our centre was introduced in 2013 and until 2017 was restricted to unsuspected adrenal tumours. Since 2018 we have started to perform laparoscopic adrenalectomy also in patients with suspected metastases, but they are not part of this analysis.
We are aware that the results of this study are burdened low numbers of patients in different groups, and statistical analysis in such small groups may be inconclusive.
It must be emphasized that the majority of studies, like the present one, are characterised by exceptionally varied study groups with a limited number of patients, which makes the results difficult to compare. Analyses of prognostic factors in case series are guided by the error of selection and bias and many variables are inter-related. All observation concentrates on adrenalectomy alone (without a discussion on additional adjuvant systemic treatment after surgery, which may affect survival). Larger pooled analyses using standardised protocols and precise definitions are necessary to identify the optimal indications for adrenalectomy for adrenal metastasis.