Introduction
„Breast cancer genes” BRCA1 and BRCA2 are by far the most widely studied human genes, and consequences of germline pathogenic variants of both genes for cancer risk are very well described [1]. Non-oncological implications of germline BRCA1 and BRCA2 genes, complicating reproductive health, including early natural menopause, reduced ovarian reserve and unresolved association between BRCA1 and BRCA2 pathogenic variants, premature ovarian failure and CGG repeat number in FMR1 gene, are far less described [2-6].
Woman’s reproductive lifespan is limited by the age of menarche and age of natural menopause (ANM). Timing of both events are determined by genetic and environmental factors, with relatively high heritability for ANM, estimated on around 50% [7]. At least intragenic 3 loci (SYCP2L, UIMC1, and MCM8) and a least 1 intergenic locus (13q34) are associated with ANM across different ethnic populations [8], and can be treated as quantitative trait loci (QTL) for ANM. Loci for premature menopause were also identified, with most widely studied association between premature ovarian failure (POF) and number of CGG repeats in FMR1 gene [9].
The influence of germinal BRCA1 and BRCA2 on AMN remains inconclusive and controversial. Hence, we conducted a comprehensive systematic review and meta-analysis of BRCA1 and BRCA2 pathogenic variants on ANM.
Material and methods
PubMed database was searched for abstracts by two reviewers (ŁK and KP) using the keywords: (“BRCA1” OR “BRCA2” OR “hereditary breast cancer”) AND (“menopause”). We identified 193 citation; both reviewers independently reviewed potentially relevant studies subsequently excluded studies which were not case-control, cohort or cross-sectional studies. Additionally, reference lists from systematic reviews or meta-analyses, dealing with the topic of menopause and BRCA1 and BRCA2 germinal pathogenic variants, were also checked to identify eligible studies. Studies dealing only with risk-reducing salpingo-oophrectomy (RRSO) and influence of ANM on breast and/or ovary cancer risk were excluded. Two studies (Table 1) not reporting dispersion measures and not reporting ANM in control group were left in tabular summary, yet were excluded from meta-analysis. Discrepancies in retrieved list were resolved by consensus. We also included our original, unpublished data from families, affected by BRCA1 or BRCA2 pathogenic variants, consisted of at least two postmenopausal female siblings with differing variant status (Table 2). None of our patients undergone RRSO prior to natural menopause. As most of the data reported median and range for ANM, we estimated mean and standard deviation using Hozo et al. approach [10]. Meta-analysis was done using random effects model on standardized mean differences. Statistical analysis was conducted using R (version 3.6.1. The R Foundation for Statistical Computing).
Table 1
Studies included in systematic review and meta-analysis
1 All cases were attributed to BRCA1 mutations, 2 range derived from Figures 3 and 4, 3 no dispersion measure nor range was given – excluded from analysis, 4 controls for BRCA1 positive group, 5 controls for BRCA2 positive group, 6 mean calculated as mean of menarche in whole group + mean reproductive lifespan, no actual data nor dispersion measure was given – excluded from analysis, 7 only one family with BRCA2 mutation
Table 2
Characteristics of BRCA1/2 positive probands and their BRCA1/2 negative siblings
Results and discussion
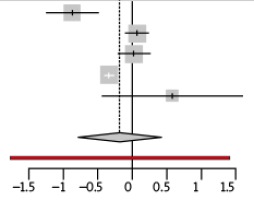
Our database search retrieved 193 articles by initial strategy, and 6 studies, combining data from 2121 germinal BRCA1 and BRCA2 pathogenic variant carriers and 3741 control subjects [11-16]. Four of the studies used Kaplan-Meier approach to assess the differences between carriers and non-carriers [12, 13, 16], two studies were excluded from meta-analysis, as they reported no dispersion measures (and we were unable unambiguously derive those data from Figures) [13] or did not report data from control group [15]. We also included original data from 7 pathogenic variant carriers and 9 non-carrier siblings, summarized in Table 2. Studies included in presented meta-analysis combined data from 1535 germinal BRCA1 and BRCA2 pathogenic variant carriers and 3191 control individuals. Results of preformed meta-analysis are presented in Figure 1. Results only from group affected with BRCA1 pathogenic variant was similar to group combining carriers of either pathogenic variants (data not shown). Shortage of data from carriers of germinal BRCA2 pathogenic variants did not enabled draw significant conclusions.
Three studies reported association BRCA1 and BRCA2 with premature menopause [11, 12, 14], two studies reported no evidence of that association [13, 16]. Meta-analysis results does not support the hypothesis of association between germinal pathogenic variants of “breast cancer genes” and premature menopause. Nevertheless, data from all included studies are prone to selection biases as cessation of observation due to RRSO or cancer-related and treatment-related menopause. Only carefully designed prospective study may resolve the true association between BRCA1 and BRCA2 and early menopause.