Introduction
Hepatocellular carcinoma (HCC) accounts for 85–90% of primary liver cancers and remains a major global health concern [1]. According to GLOBOCAN 2020, in that year liver cancer caused approximately 830,000 deaths globally, ranking as the third leading cause of cancer-related mortality [2]. While hepatic resection remains a potentially curative treatment for selected patients, long-term outcomes are compromised by high recurrence rates, reaching as high as 70% within five years [3]. This has driven research into prognostic markers to better stratify patients and optimize treatment strate-gies.
Alpha-fetoprotein (AFP) serves as an essential biomarker for HCC, recommended for diagnosis, risk stratification, and monitoring of treatment response [4, 5]. However, its clinical utility is significantly limited by the fact that 30–40% of HCC cases are AFP-negative (defined as serum AFP ≤ 20 ng/ml) [6, 7]. This challenge has led to the investigation of alternative biomarkers, including des-γ-carboxy prothrombin (DCP; also known as protein induced by vitamin K absence or antagonist-II), inflammation-based markers, and image-based radiomics, particularly for AFP-negative HCC (ANHCC) [8–10]. There have been very few studies indicating that AFP remains a prognostic factor in ANHCC, while some reports have found no significant prognostic value of AFP in this context [7, 11, 12]. Accordingly, recent prognostic models for ANHCC have incorporated conventional tumor characteristics and liver function parameters, either with or without novel biomarkers, potentially overlooking the nuanced role of AFP [13–16]. Given these inconsistencies and the limited exploration of AFP’s prognostic role in ANHCC, we aimed to:
Material and methods
Patients, data collection, and ethics statement
A total of 1,120 HCC patients who underwent hepatic resection at our institute between January 2004 and December 2023 were retrospectively identified. The inclusion criteria for this study were:
hepatic resection as the initial treatment for HCC,
preoperative serum AFP levels ≤ 20 ng/ml,
adequate preoperative liver function,
R0 resection status, irrespective of extrahepatic metastasis.
Patients were excluded if they met any of the following conditions:
received preoperative anti-HCC treatment or underwent a second or subsequent liver resection due to recurrent HCC,
histologically confirmed combined or mixed hepatocellular-cholangiocarcinoma,
presence of other active malignancies at the time of surgery,
incomplete clinical data. Ultimately, 453 patients were included in the final analysis.
Data on patient demographics, laboratory parameters, tumor characteristics, and some surgical features were extracted from medical records. The collected data included age, sex, hepatitis virus status, and liver function tests (total bilirubin, albumin, aspartate aminotransferase, alanine aminotransferase, platelet count, prothrombin activity, Child-Pugh grade, albumin-bilirubin (ALBI) score, and indocyanine green retention rate at 15 minutes (ICG-R15), as well as AFP and DCP levels, both of which were measured using a chemiluminescent enzyme immunoassay in the institution’s laboratory. The largest tumor size was assessed using preoperative computed tomography (CT), and the number of tumors was determined through a combination of imaging scans, intraoperative probing, and postoperative dissection. The Milan criterion (1 lesion smaller than 5 cm or up to 3 lesions, each smaller than 3 cm) and the up-to-seven criterion (sum of the size and number of tumors ≤ 7) were applied to evaluate tumor burden. Information on the Barcelona Clinic Liver Cancer stage [17], presence of cirrhosis as defined by a fibrosis score of 4 using the new Inuyama classification [18], Edmondson-Steiner histological grade [19], and vascular invasion (either microscopic or macroscopic) was also collected. For surgical features, the extent of resection (major resection involving three or more segments), surgical approach (open or laparoscopic), and whether concomitant ablation was performed were documented.
Data collection and analysis were conducted in accordance with the ethical standards of the 1975 Declaration of Helsinki, and the study was approved by the institutional ethics committee (approval no. 2024-28). The requirement for individual written consent in this retrospective analysis was waived due to the study’s observational nature and the use of anonymized data.
Surgical indications, procedures, and follow-up
The possibility of surgical resection was evaluated using imaging modalities such as contrast-enhanced CT, ultrasonography (US), and magnetic resonance imaging (MRI). Preoperative assessments included liver biochemistry tests, ICG-R15, and, when necessary, technetium-99m- galactosyl human serum albumin scintigraphy to assess liver function. Liver resection was pursued as monotherapy when all tumors were deemed resectable while preserving adequate remnant liver function or volume based on pre- and intraoperative evaluations. In cases where complete tumor resection was limited by liver function, combined liver resection and microwave ablation were used: larger tumors were resected, while smaller, deep-seated nodules (typically < 2 cm) underwent intraoperative US-guided ablation. When minor extrahepatic spread, such as metastases to lymph nodes or the adrenal gland, was detected pre- or intraoperatively, simultaneous resection was performed if the lesions were deemed macroscopically resectable. All procedures were conducted after obtaining written informed consent from patients. Postoperative follow-up included serum tumor marker assessments and imaging (CT, US, or MRI) initially at four weeks after surgery, followed by routine checks every three months for the first two years. Afterward, follow-up intervals were adjusted based on patient status, typically every four to six months. The endpoints of this study were overall survival (OS) and recurrence-free survival (RFS). Overall survival was measured from the date of resection to the date of death from any cause or the last follow-up. Recurrence-free survival was measured from the date of resection to the occurrence of tumor recurrence, metastasis, or the last follow-up.
Statistical analysis
The Mann-Whitney U test was used for comparing continuous variables, while the χ2 test or Fisher’s exact test was applied to categorical variables. The optimal AFP cutoff for patient stratification was determined by survival analyses at AFP thresholds between the 10th and 90th percentiles. The cutoff maximizing the product of χ2 values from both RFS and OS analyses was selected. Kaplan-Meier (KM) curves for RFS and OS were plotted using the determined AFP cutoff across the entire cohort. Patients were then randomly split into training and validation cohorts in a 7 : 3 ratio. Least absolute shrinkage and selection operator (Lasso) regression with repeated 10-fold cross-validation (20 repetitions) was employed to enhance variable selection stability, resulting in the selection of the top seven variables. These variables were further refined using backward stepwise selection based on the Akaike information criterion to finalize the Cox proportional hazards models for both OS and RFS. Nomograms were constructed based on the final models, and their performance was evaluated using concordance (C–) indices, time-dependent area under the receiver operating characteristic curves (AUCs), and calibration plots. Patients were stratified into high- and low-risk groups according to nomogram scores, with KM curves illustrating survival differences between these groups. All statistical analyses were performed using R software (version 4.2.1), with a significance level of p < 0.05.
Results
Patients’ characteristics
Table 1 summarizes the clinicopathological characteristics of the patients. The median age of the patients was 71 years, and most (79.7%) were men. Over half (52.3%) were positive for anti-hepatitis C virus antibody, and the majority (94.0%) were classified as Child-Pugh grade A. The median AFP level was 6.9 ng/ml. In terms of treatment, 68 patients (15.0%) underwent major hepatic resection, 147 patients (32.5%) received laparoscopic resection, and 33 patients (7.3%) underwent concomitant intraoperative ablation, with overlap between all three treatments. After patients had been divided into a training cohort (n = 317) and a validation cohort (n = 136), all variables were similar between the cohorts, with no significant differences.
Table 1
Patient characteristics.
[i] AFP – alpha-fetoprotein, ALT – alanine aminotransferase, AST – aspartate aminotransferase, BCLC – Barcelona Clinic Liver Cancer, DCP – des-γ-carboxy prothrombin, HBs-Ag – hepatitis B virus antigen S, HCV-Ab – anti-hepatitis C virus antibody, ICG-R15 – indocyanine green retention rate at 15 minutes
Alpha-fetoprotein levels and prognosis in recurrence-free survival and overall survival
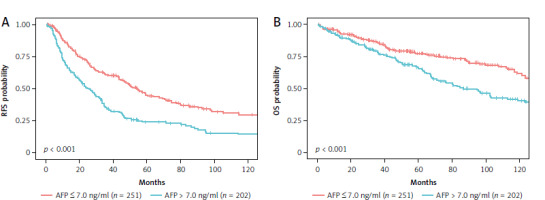
Initially, the prognostic significance of AFP levels for both RFS and OS was assessed by stratifying all patients into high AFP (n = 202) and low AFP (n = 251) groups using an optimal cutoff value of 7.0 ng/ml. During the median follow-up time of 54.8 months (95% CI: 47.8–62.2 months), patients in the high AFP (> 7 ng/ml) group had a significantly higher risk of recurrence compared to those in the low AFP (≤ 7 ng/ml) group, with a hazard ratio (HR) of 1.79 (95% CI: 1.41–2.27, p < 0.001) (Fig. 1 A). Similarly, for OS, high AFP levels were associated with a greater risk of mortality, with a HR of 1.64 (95% CI: 1.22–2.20, p < 0.001) (Fig. 1 B).
Development and validation of the nomogram for recurrence-free survival
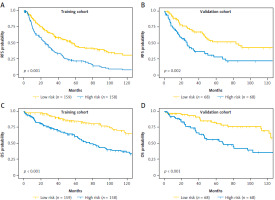
In the training cohort, Lasso regression was performed using 22 clinicopathological variables listed in Table 1. This analysis identified variables with the highest coefficients, including AFP, age, sex, anti-hepatitis C virus antibody positivity, prothrombin activity, multiple tumors, and cirrhosis. Stepwise Cox regression subsequently selected AFP, age, sex, multiple tumors, and cirrhosis as independent prognostic factors for RFS (Table 2), which were then used to develop the nomogram (Fig. 2 A). The nomogram demonstrated a C-index of 0.64 (95% CI: 0.60–0.68) in the training cohort, with an AUC ranging 0.65–0.74 for up to 10 years after surgery (Fig. 3 A). In the validation cohort, the C-index was 0.64 (95% CI: 0.62–0.65), and the AUC was in the range 0.64–0.76 (Fig. 3 A). Calibration plots for RFS at 1, 3, and 5 years in both cohorts closely aligned with the ideal line, indicating good predictive accuracy (Figs. 4 A, B). Further stratification based on the median nomogram score classified patients into high- and low-risk groups. In the training cohort, the HR for the high-risk group was 2.10 (95% CI: 1.59–2.79; p < 0.001) (Fig. 5 A), and in the validation cohort, it was 2.08 (95% CI: 1.30–3.30; p = 0.002) (Fig. 5 B), demonstrating strong discriminatory power for RFS.
Table 2
Multivariable analysis of prognostic factors using Cox stepwise backward selection in the training cohort
Fig. 2
Nomograms for recurrence-free survival (A) and overall survival (B)
AFP – alpha-fetoprotein, ALBI score – albumin-bilirubin score, DCP – des-γ-carboxy prothrombin, OS – overall survival, RFS – recurrence-free survival
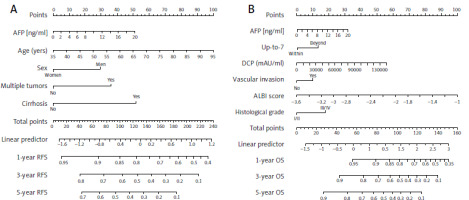
Fig. 3
Time-dependent area under the receiver operating characteristic curves for recurrence-free survival (A) and overall survival (B) in the training and validation cohorts
AUC – area under the receiver operating characteristic curves, OS – overall survival, RFS – recurrence-free survival
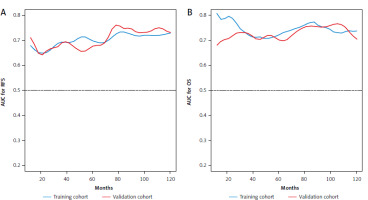
Fig. 4
Calibration plots for recurrence-free survival (RFS) and overall survival (OS) at 1, 3, and 5 years in the training and validation cohorts: RFS in the training cohort (A), RFS in the validation cohort (B), OS in the training cohort (C), and OS in the validation cohort (D)
OS – overall survival, RFS – recurrence-free survival
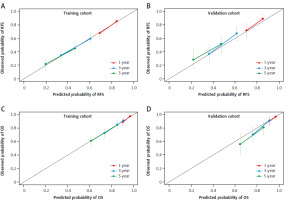
Fig. 5
Kaplan-Meier survival curves for recurrence-free survival (RFS) and overall survival (OS) stratified by the median nomogram score into high- and low-risk groups in the training and validation cohorts: RFS in the training cohort (A), RFS in the validation cohort (B), OS in the training cohort (C), and OS in the validation cohort (D)
OS – overall survival, RFS – recurrence-free survival
Development and validation of the nomogram for overall survival
For OS, repeated Lasso regression identified variables with the highest coefficients: AFP, prothrombin activity, ALBI score, DCP, the up-to-7 criterion, vascular invasion (either microscopic or macroscopic), and histological grade. Stepwise Cox regression was then applied to optimize a model using these variables, resulting in a final model that included AFP along with five other predictors (Table 2). Although some variables, such as vascular invasion and histological grade, were not statistically significant, AFP remained an independent prognostic factor for OS. This optimized model was subsequently used to develop the OS nomogram (Fig. 2 B). The overall survival nomogram demonstrated a C-index of 0.72 (95% CI: 0.67–0.76) in the training cohort, with an AUC in the range 0.71–0.81 over a 10-year postoperative period (Fig. 3 B). In the validation cohort, the C-index was 0.67 (95% CI: 0.65–0.68), with an AUC range of 0.67–0.77 (Fig. 3 B). Calibration plots for 1, 3, and 5-year OS in both cohorts closely matched the ideal line, indicating good predictive accuracy (Fig. 4 C, D). When stratified by the median nomogram score, patients were effectively categorized into high- and low-risk groups. In the training cohort, the high-risk group had a HR of 3.00 (95% CI: 2.05–4.39; p < 0.001) (Fig. 5 C), while in the validation cohort, the HR was 2.65 (95% CI: 1.52–4.63; p < 0.001) (Fig. 5 D), demonstrating robust discriminatory power for OS.
Discussion
In this study, we demonstrated that AFP was an independent prognostic factor for both RFS and OS in ANHCC patients following curative resection. Alpha-fetoprotein, a glycoprotein synthesized by fetal hepatocytes and yolk sac cells, was first identified as a biomarker for HCC in the 1960s [20]. The prognostic significance of AFP has been recognized by several scoring systems, including the BALAD score, the Cancer of the Liver Italian Program score, and the Chinese University Prognostic Index, although the AFP cutoff values accepted in these systems are relatively high, in the range 400–500 ng/ml [21–23]. Not only is alpha-fetoprotein a well-established tumor marker for HCC, but it is also well known that mild elevation can occur in patients with chronic liver diseases such as hepatitis and cirrhosis due to liver regeneration [20]. This increase may indicate a higher risk of HCC, as persistent AFP production could reflect subclinical tumor activity or abnormal hepatic cell proliferation [24]. Recent evidence suggests that AFP is not merely a passive tumor marker but may actively contribute to hepatocarcinogenesis by enhancing liver cancer cell proliferation, promoting immune evasion, and stimulating angiogenesis, thereby facilitating tumor progression [25, 26]. In a large cohort study by Lin et al., elevated AFP levels (≥ 4.6 ng/ml) were linked to adverse features, including multiple tumors, poor differentiation, and microvascular invasion, even among patients with ANHCC who underwent curative resection [7]. Differences in AFP cutoffs between their study and ours may reflect differences in patient populations and study methodologies, underscoring the need to contextualize findings within specific study populations. Blank et al. further demonstrated that categorizing AFP into quintiles revealed progressively worse outcomes as AFP levels increased, with patients in the first quintile (1.4–4.1 ng/ml) showing better survival outcomes than those in higher quintiles [11]. Thus, even at clinically normal levels, AFP may still serve as a sensitive indicator of subtle oncogenic processes, supporting its integration into prognostic models for ANHCC.
As far as we are aware, this is the first study to develop a nomogram that integrates AFP for patients with ANHCC. By combining Lasso regression for variable selection and Cox proportional hazards modeling, we were able to build a prognostic tool for both RFS and OS in this cohort. The use of Lasso regression followed by stepwise Cox regression allows for efficient variable selection, reducing model complexity while minimizing overfitting and addressing multicollinearity—a crucial aspect in HCC, where multiple prognostic factors are present [27]. Previous studies have developed prognostic nomograms for ANHCC patients undergoing hepatic resection using various clinicopathological factors [13–16]. For instance, Yang et al. included platelet-ALBI score, tumor size, and microvascular invasion for RFS prediction, while Wang et al. focused on OS using body mass index, tumor stage, metastasis stage, and hematological markers [13, 14]. More recently, Yan et al. incorporated CT radiomics features along with traditional clinicopathological factors to predict early recurrence [10]. The diversity in variables selected by models was likely due to differences in patient populations, institutional practices, and statistical techniques. However, our nomogram still demonstrated acceptable discriminatory performance, uniquely incorporating AFP as a prognostic marker.
In addition to AFP, DCP was selected as a predictor in our OS nomogram. Both AFP and DCP are important biomarkers for HCC prognosis, but they may have different implications in predicting outcomes. In particular, DCP tends to be elevated in more advanced HCC, such as those with large tumor size and vascular invasion, highlighting its association with aggressive disease characteristics [28, 29]. Qiu et al. recently developed a scoring model combining DCP and tumor burden score, which successfully predicted OS and early recurrence following resection of ANHCC [8]. However, the contribution of DCP to our OS nomogram may be less pronounced due to the observed range of values. Although the median DCP level in the overall and training cohort was 45 mAU/ml, the nomogram scale extended up to 130,000 mAU/ml, reflecting a highly skewed distribution where only very high values of DCP would significantly influence the final score. This suggests that while DCP remains an important marker, its practical contribution to the nomogram might be limited when its levels are not substantially elevated.
Several limitations must be considered when interpreting the results of this study. First, this was a single-center, retrospective analysis with a relatively small sample size, and we did not have access to an independent external validation cohort. Second, our nomograms included histological parameters that can only be assessed postoperatively, thus limiting their applicability for preoperative decision-making. Third, while we focused on developing a prognostic model for RFS and OS, long-term outcomes can be influenced by various factors not captured in our analysis, such as comorbidities, lifestyle habits, postoperative complications, recurrence patterns (e.g., intrahepatic vs. extrahepatic), and serum AFP dynamics after recurrence, which were beyond the scope of this study. Furthermore, although our nomograms demonstrated acceptable discriminatory performance, their C-indices (0.64 for RFS and 0.67–0.72 for OS) were modest. This moderate predictive accuracy may reflect the inherent challenge in prognostication for ANHCC patients, who represent a more homogeneous population compared to the general HCC population. Future studies incorporating additional biomarkers or molecular features might help improve the predictive performance of these models. Lastly, the study period spanned several years, during which changes in surgical techniques, postoperative management, and advancements in drug therapies have occurred, introducing potential temporal bias that could affect the applicability of our findings to current clinical practice.
Conclusions
This study demonstrated that AFP retained significant prognostic value for ANHCC patients undergoing hepatic resection. Integrating AFP into nomograms, alongside other clinicopathological variables, offers a valuable tool for predicting both RFS and OS. These nomograms improve risk stratification and support personalized follow-up and surveillance strategies in the post-resection setting for ANHCC.