Introduction
Endometrial cancer (EC) is one of the most common types of gynaecological malignancies worldwide [1]. The most prevalent histopathological type of EC is endometrioid endometrial cancer (EEC), which accounts for 80% of all cases of ECs [2]. A diagnosis of EC is usually made in the early stages of disease (I and II, according to the International Federation of Gynaecology and Obstetrics – FIGO) when the prognosis tends to be more favourable [3]. In more advanced stages of the disease the treatment requires a multimodal approach, and the prognosis is poor [4]. Prognostic factors in cases of EC include: age, histopathological subtype, tumour grading, myometrial and cervical involvement, the presence of lymph vascular space invasion, and the presence of lymph node or distant metastases [5, 6]. In the group with lymph node metastases (LNM), the number of metastatic lymph nodes among all dissected nodes, known as the lymph node ratio (LNR), may be an even more sensitive predictor of patient survival [7, 8].
In most cases the radical treatment of EC involves surgery [9]. Lymphadenectomy improves the accuracy of surgical staging and helps in assessing the extrauterine spread of the disease; thus, it allows for adjuvant therapy to be planned more precisely. When an adequate lymphadenectomy is performed and LNM is not detected, more patients can avoid additional therapy, which means that radiotherapy- and chemotherapy-related morbidity can be reduced. On the other hand, lymphadenectomy is associated with a higher incidence of surgery-related adverse events [10–12].
The therapeutic role of lymphadenectomy in the management of EC remains controversial. So far only 2 prospective, randomized studies on the therapeutic effect of lymph node dissection in patients with EC have been conducted, and their results do not support the existence of such a therapeutic effect in low-risk patients [10, 13]. On the other hand, a number of retrospective studies have shown that the therapeutic role of lymphadenectomy may depend on the total number of dissected lymph nodes [4, 14–16].
Since 2009, the assessment of pelvic and paraaortic lymph nodes (PALN) has been included separately in the FIGO classification [17]. Patient survival is strongly influenced by the presence of LNM. Nevertheless, cases with node-positive disease constitute a heterogeneous group because the estimated 5-year disease-specific survival rates vary from 10 to 75% [7, 18–20]. The resection of metastatic lymph nodes is part of the radical treatment of EC [9]. However, the precise impact of LNM – with consideration of such features as the total number of nodes, their localization, and their character – on patient survival has yet to be established [19, 21–23].
Consequently, the primary aim of our study was to analyse the impact of selected features of LNM, such as the LNR, the presence of extracapsular involvement (ECI), the total number of lymph nodes involved, and the localization of LNM, on EC patient prognosis.
Material and methods
We carried out a retrospective study of patients who underwent surgical treatment for endometrial cancer in the 2nd Department of Obstetrics and Gynaecology, Centre of Postgraduate Medical Education in Warsaw, Poland, and in the Clinical Division of Gynecological Oncology of the Franciszek Lukaszczyk Oncological Center in Bydgoszcz, Poland. Patients’ medical records, including demographic and histopathological features, as well as treatment and follow-up details, were analyzed. Women underwent either laparoscopy or longitudinal laparotomy and total hysterectomy with bilateral salpingo-oophorectomy between 2000 and 2015. The final histopathological results were based on World Health Organization guidelines. Only those patients with endometrial cancers were included in this research. Disease staging was assessed using the FIGO 2009 staging system [24]. Patients treated before 2009 were reclassified according to the FIGO 2009 staging system.
In each case, the histopathological subtype as well as the tumour stage and grade were assessed. Adjuvant treatment was based on the current National Comprehensive Cancer Network guidelines and, accordingly, included vaginal brachytherapy, external beam radiation therapy, and chemotherapy. Low-risk patients did not receive adjuvant treatment and remained in follow-up. Overall survival (OS) was calculated from the date of surgery to the date of death or last follow-up.
For the subgroup of patients with metastases to lymph nodes, we evaluated the impact of the number and localization of metastatic lymph nodes, the LNR, and the ECI on OS. Patient survival was presented as both 5-year overall survival (5-year OS) and median OS (mOS). The LNR was defined as the ratio of the number of positive lymph nodes to the total number of dissected lymph nodes.
The relationship between the FIGO and tumour grade with LNM was calculated using the Fisher exact test with the Freeman-Halton extension for the 3 × 2 and 4 × 2 table. Information on any patients who died was retrieved from the database of the National Health System of Poland. Survival analyses were conducted using Kaplan-Meier survival curves, and the differences in patient survival were compared using the log-rank test. To evaluate the impact of LNR, we performed a multivariate survival analysis using Cox proportional-hazards regression with a stepwise entry method, and we analysed the LNR cut-offs incrementally at 0.05 intervals, starting at 0.05, and then at 0.1, 0.15, 0.2, and so on, up to and including 0.95.
We identified 866 patients who underwent surgical treatment for EC. Of these patients, 215 (24.8%) had both pelvic and paraaortic lymphadenectomy, 507 (58.5%) had only pelvic lymphadenectomy, and one had only para-aortic lymphadenectomy. Patients who did not undergo lymphadenectomy, who were suffering from non-endometrioid endometrial cancer, or for whom it was not possible to obtain follow-up information, were excluded from the analysis (n = 229). Consequently, the study group included 637 EC patients.
Results
The median age of patients who underwent pelvic lymphadenectomy, paraaortic lymphadenectomy, or both was 60 years (IQR 56–68). The median number of removed pelvic lymph nodes (PLN) was 13 (IQR 8–19) whereas the median number of removed PALN was 7 (IQR 4–11).
Of the 637 patients evaluated, 75 (11.7%) were diagnosed with LNM; 562 (88.2%) did not have LNM. Of the 75 identified cases, pelvic LNM were found in 71 (94.7%) cases, while paraaortic LNM were present in 17 (22.7%) cases, and 7 (9.3%) cases were histopatholo-gically diagnosed with ECI. When we focused on those patients who had both pelvic and paraaortic lymph node dissection (n = 198 cases), LNM were found in 31 (15.7%) cases. Within this subgroup there were 13 (41.9%) cases of posi-tive pelvic and paraaortic lymph nodes; 15 (48.4%) cases had positive pelvic lymph nodes only, and 3 (9.7%) cases were found with isolated metastases in paraaortic lymph nodes. Therefore, paraaortic LNM were found in 16 patients (51.6%) with LNM. However, when all patients were included (also patients without LNM who had pelvic and paraaortic lymph node dissection, n = 198), the rate of isolated paraaortic LNM was 1.5%. LNM were more often present in patients with more advanced disease (Table 1). We also observed a higher rate of poorly differentiated tumours in the group of EC patients with LNM (Table 1). We found no difference in age between patients with and without LNM (Table 1).
Table 1
Clinicopathologic characteristics of endometrioid endometrial cancer patients with and without lymph node metastases
We observed significant differences in the median survival rate of EEC patients without LNM compared to patients with LNM and to those with both LNM and ECI (p < 0.0001). The 5-year overall survival rate in the group of patients without LNM was 87% (mOS was not reached, range 1.7–200 months), while in patients with LNM, the 5-year OS rate was 64% (mOS = 144 months, range 0.2–175 months). The group of patients with ECI included only 7 women, and within this group the 5-year OS rate was 43% (mOS was not reached, range 2–125 months) (Fig. 1 A).
Fig. 1
Survival of endometrioid endometrial cancer (EEC) patients according to lymph node metastases (LNM). Group 0: patients (n = 562) without LNM; Group 1: patients (n = 68) with LNM; Group 2: patients (n = 7) with extracapsular involvement (ECI); (p < 0.0001) (A), Survival of EEC patients according to the site of lymph node metastases. Group 1: patients (n = 15) with only pelvic lymph node metastases; Group 2: patients (n = 16) with paraaortic lymph node metastases; (p = 0.26) (B), survival of EEC patients according to lymph node ratio (LNR). Group 1: patients (n = 51) with a LNR < 0.3; Group 2: patients (n = 24) with LNR ≥ 0.3; (p = 0.003) (C), survival of EEC patients with LNM according to the extension of the primary tumour (T parameter from TNM classification). Group 1: patients (n = 49) with T1 tumour; Group 2: patients (n = 22) with T2 tumour; Group 3: patients (n = 3) with T3 tumour; (p = 0.008) (D)
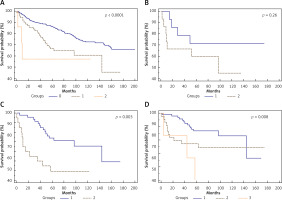
In the group of patients who had both pelvic and paraaortic lymphadenectomy, the 5-year OS rate in the group of patients with paraaortic LNM was 60% (mOS was not reached, range 4.7–135.3 months), while the 5-year OS rate in the group of patients with only pelvic LNM was 71% (mOS not reached, range 31 months, range 0.2–175 months). However, the difference was not statistically significant (p = 0.26) (Fig. 1 B).
In the group of patients with LNM, the multivariant survival analysis indicated that 0.3 was the best LNR cut-off for differentiating between short- and long-term survivors, with a hazard ratio for death of 2.94 (95% CI: 1.49–5.80). Patients with a LNR ≥ 0.3 (n = 24) had a significantly shorter OS period (35.0 months, range 0.2–175 months) compared to patients with a LNR < 0.3 (n = 51) (mOS was 143, range 15–169 months; p = 0.003) (Fig. 1 C). The 5-year OS rates for patients with LNR ≥ 0.3 and < 0.3 were 39% and 71%, respectively.
In the group of patients with LNM, we found that a poorer prognosis depended on the extension of the primary tumour (T parameter from TNM classification: T – tumour, N – nodus, M – metastases; p = 0.008). Patients with LNM and T1 tumour, according to TNM classification, had a 5-year OS rate of 86% (mOS = 143 months, range 18–169 months). Patients with T2 tumour had a 5-year OS rate of 44% (mOS = 63 months, range 0.2–175 months), while in the cases involving patients with T3 tumour, none of the patients survived 5 years (mOS = 43 months, range 4–43.6 months) (Fig. 1 D). Due to the limited number of samples, patients with T4 tumour were excluded from the analysis.
Discussion
The LNR is considered a useful prognostic tool in cases involving various types of neoplasms [25–28]. In the largest currently available report concerning patients with EC, Chan et al. divided EC patients into 3 groups according to the percentage of positive lymph nodes in relation to the total number of dissected nodes: ≤ 10, 10–50, and ≥ 50%. The reported 5-year disease-specific survival (DSS) rates were as follows: 77.3%, 60.7%, and 40.9%, respectively. Regarding the total number of positive lymph nodes, the 5-year DSS for women with 1, 2–5, and ≥ 5 lymph nodes was 68.1%, 55.1%, and 46.1%, respectively [7]. The higher the number of dissected lymph nodes, the better the observed outcome with respect to the LNR. Furthermore, it was discovered that among patients who had fewer than 10 lymph nodes removed, the LNR was no longer a good prognostic factor. In this subgroup only the total number of metastatic lymph nodes can be used as a prognostic tool [7]. Similarly, Polterauer et al. reported a decrease in the 5-year OS with an increase of the LNR for patients who had at least 10 lymph nodes dissected. The rate was 79% for LNR ≤ 10, 61% for LNR 10–50, and 36% for LNR ≥ 50% [8]. Additionally, we observed that a high LNR is a prognostic factor for OS. However, in our group, LNR ≥ 0.3 (32%) was the best cut-off value. We also showed that the higher the total number of metastatic lymph nodes, the poorer the OS, and this correlation was observed especially in cases involving more than 2 metastatic lymph nodes. Although LNR might be a good predictor of poor long-term outcomes for EC patients, lymph node dissection is necessary for assessing LNR, and the accurate identification of LNM depends on the extension of the lymphadenectomy. Consequently, these results should be considered when planning surgical treatment for patients with EC.
In this retrospective analysis, we observed a significant difference in the mOS between patients with LNM compared to patients without nodal involvement. Lymph node metastases constitute a well-known poor prognostic factor for patients with EC [19, 29]. Furthermore, we showed that the prognosis was worse in cases where there was an increase in the number of lymph nodes involved and the presence of the ECI. The data on the clinical significance of ECI in EC patients is limited but seems to support our findings [30, 31]. It has been hypothesized that the loss of capsular integrity may be a symptom of a more aggressive tumour, weaker host immune response, and poorer response for adjuvant therapy [31]. Furthermore, our observations indicate that lymph nodes with ECI are more difficult to remove during surgery. Although the analysed group consisted of 637 cases, the number of cases with ECI was only 7. We can conclude that the loss of capsular integrity is not frequent, and further studies with a larger sample size are needed to confirm the clinical impact of ECI.
The difference in the survival rates between patients diagnosed as stage IIIC1 and those diagnosed as stage IIIC2 is controversial. We noticed a trend towards a worse prognosis for patients with paraaortic LNM compared to those patients with only pelvic LMN; however, the difference was not statistically significant. Similar results were obtained by Todo et al., who analysed the following 4 groups of EC patients: 1) patients without LNM; 2) patients with pelvic but not paraaortic LNM; 3) patients with both pelvic and paraaortic LNM; and 4) patients with isolated paraaortic LNM. As we observed in our study, the authors found a trend towards a worse prognosis for EC patients with paraaortic LNM; however, the difference was not statistically significant [32]. Similar results were obtained by McMeekin et al., [33]. However, other studies reported significantly poorer prognoses for EC patients with paraaortic lymph node metastases, especially when an increasing number of positive PALN was found [33–35].
However, it should be noted that not only the localization of LNM, the presence of ECI, and high LNR are related with poor patient prognosis. Previously, Mariani et al. divided IIIC stage patients in 2 subgroups: 1) with nodal involvement only and 2) with additional cytologic, serosal, adnexal, or vaginal involvement. Their results revealed poorer prognosis for those with more advanced extent of primary tumour [20]. We also observed that among the patients with LNM the local advancement of the disease (T parameter from TNM classification) was related with patient survival.
In our study, we observed that 15.7% of patients who underwent lymphadenectomy (pelvic or pelvic and paraaortic) during primary surgery for EECs were diagnosed with LNM. However, in the group of patients with only pelvic lymph node dissection, LNM were found in 11.7% of patients. Our results are similar to those of previous studies [36–38]. However, data from the Surveillance, Epidemiology, and End Results on 19,329 EC patients reveal that lymph node involvement may occur only in 5.3% of surgically treated women [39]. When limited to at-risk patients (grade 3, MI > 50%, and primary tumour diameter > 2 cm), lymphadenectomy may lead to a higher rate of LNM, up to 10–22% [39, 40]. The patients included in this analysis comprised a heterogenous group with respect to tumour grade and TNM classification, but we also observed a higher prevalence of LNM in the group of patients with poorly differentiated tumours and more advanced disease.
In our study, the frequency of isolated paraaortic LNM was 9.7% among all EEC patients with LNM; in previous trials the frequency ranged from 4.5 to 17% [29, 33, 34, 37, 40]. The incidence of isolated paraaortic LNM among all ECC patients with pelvic and paraaortic lymph node dissection was 1.5%, which is similar to the incidence rate observed in previous studies [41]. These data suggest that pelvic lymphadenectomy without paraaortic lymph node dissection may result in an underdiagnosis of the disease stage only in about 2% of cases. However, the overall incidence of paraaortic LNM in FIGO stage IIIC patients was 51.6%, which, as previously mentioned, is high [33]. Our observation is similar to previous reports that revealed that if the PLN were positive, the likelihood of positive PALN ranged from 38 to 51% [33, 36, 40].
The main limitation of our study was the retrospective character of the analysis. We cannot exclude selection bias, and we could rely only on previous collected and designed data. Additionally, we did not evaluate the impact of adjuvant treatment and comorbidities on patient prognosis. On the other hand, our sample size was large, the follow-up period was long, and our study was based on the analysis of patients’ overall survival.







