Introduction
Chronic obstructive pulmonary disease (COPD) is a health problem with high morbidity and mortality worldwide [1]. In the case of emphysema, lung and alveolar tissues are destroyed and as a result of hyperinflation dead cavities developing in the parenchyma. Hyperinflation is the main pathophysiological mechanism of pulmonary emphysema leading to dyspnea and poor quality of life [2]. The standard measure is for patients to quit smoking. As a medical treatment, inhaled long-acting bronchodilators, inhaled steroids, oral bronchodilators, pulmonary rehabilitation, optimal nutrition and vaccination are applied [3]. Lung volume reduction procedures are accepted as the gold standard treatment in patients where medical treatment is not sufficient and cannot provide optimal recovery [4].
Lung volume reduction surgery (LVRS) and bronchoscopic lung volume reduction (BLVR) using valves or coils are recommended by the Global Initiative for Obstructive Lung Disease (GOLD) [5]. The advantage of LVRS over medical treatment is more important in patients with upper lobe dominant emphysema and low exercise capacity after rehabilitation. On the other hand, lung volume reduction is reported to improve the quality of life in patients with advanced and heterogeneous emphysema [6].
LVRS, defined for the first time in 1957, was performed through a thoracotomy incision. It was defined as resection of the most emphysematous parts of the lung [7]. However, now lung volume reduction surgery is applied in a more minimally invasive manner with the development of video-assisted thoracoscopic surgery (VATS) methods. The most important reason for this is that VATS is used in many procedures including major lung resections in thoracic surgery operations. In addition, VATS provides a wide field of view in lung and mediastinal surgery [8, 9]. With the use of a single port or multiple ports, patients have advantages over thoracotomy in terms of postoperative pain [9, 10]. Since it has a lower postoperative pain rate, the time to return to daily life in patients undergoing LVRS is shortened. Although LVRS is more invasive than bronchoscopic lung volume reduction procedures, LVRS has been shown to reduce the risk of emphysema-induced mortality beyond symptomatic relief [11]. Both methods have advantages and disadvantages. Therefore, it is important to personalize the treatment in emphysema patients. When planning the treatment preference, evaluation of patients by a multidisciplinary team and taking the LVRS decision accordingly are recommended in many studies.
Aim
In our study, we aimed to evaluate the results of patients who underwent lung volume reduction surgery with VATS due to diffuse or upper lobe limited emphysema.
Material and methods
Twenty-six patients who underwent LVRS for emphysema in our clinic between March 2015 and January 2020 were included in the study. The files of the patients were evaluated retrospectively. In the patient records, age, gender, smoking history, hospitalization time, drain removal time, complications, preoperative and postoperative pulmonary function test values were recorded. Those with chronic comorbid diseases such as coronary artery disease, uncontrolled diabetes, or uncontrolled hypertension were not included in the study. Inclusion and exclusion criteria were determined in the study (Table I). Prior informed consent was obtained from all patients. Before the operation, all patients underwent bilateral chest radiography, thorax computed tomography (CT), and pulmonary function test. There was no DLCO test in our hospital, so patients could not be evaluated with this test.
Table I
Selection criteria for lung volume reduction surgery
The surgical procedure was started with VATS in all patients, but patients who continued with thoracotomy due to severe adhesion were excluded from the study. The lung tissue targeted for resection was decided by preoperative radiological evaluation and peroperative exploration. These lung areas were excised with a standard 60 mm endoscopic staple. At the end of the operation, 1 chest tube was routinely placed. Patients were routinely left to passive drainage by chest tube, but patients with excessive air leakage and an expansion defect on chest X-ray on the first postoperative day were subjected to negative aspiration. If the air leak after surgery continued for more than 7 days, it was considered as a prolonged air leak. The patients were routinely given mucolytic drugs and nebulization therapy. For pain control, non-steroidal anti-inflammatory agents and narcotic analgesia drugs were used when necessary. Perioperative prophylactic antibiotics were continued for at least 5 days or until the chest tube was removed. Pain of patients was determined by VAS scoring and recorded. In the postoperative period, patients were followed up with daily chest radiographs. When necessary, control was done with blood tests.
Results
Twenty-four of the patients were male and 2 were female. The average age was determined as 49.6 ±16.4 (32–68). Twenty-six patients underwent 31 surgical procedures, 5 of which were bilateral. Twenty-seven of them were performed by videothoracoscopic LVRS. Surgery could not be performed in 1 patient due to pleural adhesion, and this patient was excluded, and patients who underwent LVRS with thoracotomy were not included in the study. The average cigarette consumption in our patients was 36.1 packs/year, but all patients quit smoking before the operation.
Preoperative mean forced expiratory volume in 1 s (FEV1) value was 1.2 l and 32.7%, total lung capacity (TLC) 8.6 l (132%), residual volume (RV) 7.3 l (280%). For the purpose of controlling air leakage during surgery, fibrin tissue adhesives were used in 11 (42.3%) cases, bioabsorbable staple line reinforcement material was applied in 6 (23%) cases, and leakage control was achieved with absorbable biomaterial cover in 7 (27%) patients. The staple area was supported with pleural tents in 3 (11.5%) patients, and apical pleural decortication was performed in 4 (15.3%) patients. In patients who underwent pleural tents, the drain was placed over the pleural tent (Figure 1). The average length of hospital stay was found to be 7.75 days (4–19), and the time to removal the thorax drain was 9.5 (4–23) days. The demographic status of the patients and their perioperative findings are presented in Table II. Patients with excessive air leakage in the postoperative period were subjected to negative aspiration with –20 mm H2O following surgery.
Figure 1
Preoperative chest X-ray (A) and thorax CT image (B) of the patient who underwent right LVRS 3 years ago. C – Postoperative chest X-ray of the same patient after left LVRS and pleural tents
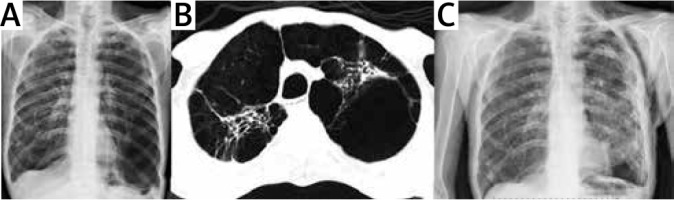
Table II
Baseline demographics and perioperative results
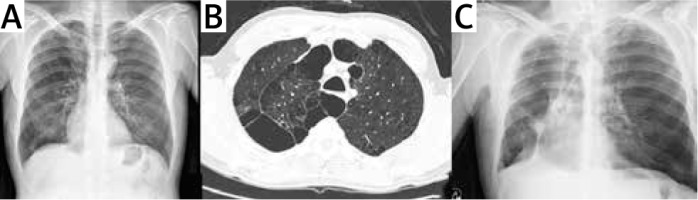
Patients whose hospitalization was prolonged, lung expansion was not completed, but the air leak was reduced or not, they were discharged by installing a Heimlich valve in the thorax drain. The longest drainage period was found to be 23 days. Two patients underwent LVRS together with cancer surgery due to upper lobe originating squamous cell carcinoma (Figure 2). One of them was left upper lobectomy and the other was right upper lobectomy. The postoperative VAS score of the patients was found to be 2.7 on average.
Figure 2
Images of the patient undergoing right upper lobectomy due to lung cancer and emphysematous lung disease, preoperative chest X-ray (A) and thorax CT (B), chest X-ray at the second postoperative month (C)
In the early period, 53.8% of the patients developed complications; the most common complication was prolonged air leakage (9 patients, 34.6%). Due to the prolonged air leak, 2 (7.7%) patients underwent reoperation and air leak repair. Pneumonia (2 patients, 7.7%) and atrial fibrillation (1 patient, 3.8%) were observed (Table III). Mortality was not observed at the end of the first postoperative month; 1 (3.8%) patient died at the 8th month due to pneumonia and COPD exacerbation. FEV1: 1.78 (48.5%) was found in the pulmonary function tests of the patients at the 6th month postoperative controls. According to preoperative FEV1, 48.3% improvement was detected and this value was statistically significant (p < 0.05).
Discussion
Lung volume reduction surgery, as seen in our study, gives good results in suitable patients in the long term. Morbidity and mortality rates in patients are also low because it can be applied minimal invasively with videothoracoscopic surgery. In NETT, one of the important studies in the field of emphysema, patients eligible for surgery were shown to give better results in patients with FEV1, less than 45%, TLC: higher than 100%, RV: higher than 150% [12]. In the same study, previous LVRS surgery, heterogeneous emphysema and pulmonary hypertension were accepted as exclusion criteria [12]. In our study, while selecting patients for surgery, NETT criteria were taken as the basis and surgery was planned for patients with FEV1 less than 45%. The mean preoperative FEV1 value of the patients was 32.7%. In the study of Ginsburg et al. [13] containing 90 patients, the mean FEV1 value was reported to be 25.8%, and we see that the results of our study are consistent with the literature.
In many reports in the literature, the rates of males and females are similar to each other in patients undergoing LVRS [14, 15]. However, in our patient series, male patients accounted for 92.3% of our sample, and we think that this is due to the high rate of male smoking in our country. All of our patients were smokers, but all patients scheduled for surgery had stopped smoking at least 4 weeks before surgery. Medical mucolytic treatments were arranged, and respiratory physiotherapy was applied to the patients in the postoperative period, accompanied by a specialist physiotherapist.
There are many studies proving that the use of fibrin tissue adhesive prevents air leakage during lung resections [16]. The risk of air leakage is higher, especially after LVRS procedures that are surgically applied due to the emphysematous lung. In addition, long-term postoperative air leaks are associated with prolonged hospitalization times, increased hospital costs, and increased incidence of cardiopulmonary complications [17]. This increases the morbidity and mortality. Since we know this situation, fibrin tissue adhesives were used in 11 cases to prevent prolonged air leakage in patients with high risk of air leakage. Prolonged air leakage was observed in 9 patients in the postoperative period and only 2 of these patients were used with fibrin tissue adhesive. Of the eleven fibrin adhesives, 4 of them were used in combination with bioabsorbable staple line reinforcement material and 5 of them were combined with bioabsorbable cover, and no prolonged air leakage was observed in these patients.
In our study, bioabsorbable staple line reinforcement material was used in 6 patients (4 patients combined with fibrin glue) and no prolonged air leakage was observed in these patients. However, since there were not enough patients to compare, it was not determined whether it was statistically significant. It is known that the combined use of fibrin adhesive and staple line supporting materials is an effective air leak control method [17]. In the study of Murray et al. [17], it was observed that staple line supportive materials reduced the rate of air leakage. At high airway pressures, the ePTFE material has been reported to have a superior effect compared to the patch made of bovine pericardium. In their study on 66 patients undergoing lung resection, Thomas et al. [18] showed that staple line supportive material made from knitted calcium alginate prevented air leakage in 55% of patients. As a result of this phase 2 study, knitted calcium alginate bioabsorbable material has been reported to be ergonomic and safe [18]. This indicates that we need to do a randomized controlled study for prolonged air leakage.
Another air leak prevention method used during lung volume reduction surgery is pleural tent and pleural decortication [19, 20]. It is known that with the application of pleural tenting after upper lobectomy/bilobectomy, the duration of intrapleural drainages and hospital stay is significantly reduced. It is a simple, safe and effective supplementary method in the prophylaxis of permanent alveolar air leakage and apical residual pleural space formation. In randomized controlled studies of Petrov et al. [21] including 40 patients, it was recommended that pleural tent application be applied prophylactically for alveolar air leakage control. In our series, pleural tents were applied in 3 of our patients, and pleural decortication in 4 of our patients. No fibrin glue or staple liner supportive biomaterial was used in these patients. However, prolonged air leakage was observed in one of our patients, and this patient was reoperated and air leak repair was performed.
Another interesting observation among our patients is that we have 2 patients who were followed up for a diagnosis of non-small cell lung cancer and underwent upper lobectomy. These patients are those in whom major lung resection is difficult due to the emphysematous lung. However, especially in patients with emphysema located in the upper lobe, it is a surgical advantage that the tumor is located in the upper lobe. The combination of major lung resection with LVRS provides an opportunity for selected patients with early-stage lung cancer and severe emphysema to undergo cancer resection rather than further reduction in pulmonary function. In these 2 patients, where we performed upper lobectomy (right upper and left upper lobectomy), no respiratory distress was observed in the postoperative period. Choong et al. [22] also recommend concomitant lung resection and LVRS surgery. In a study conducted by Caviezel et al. [23], it was reported that sublobar resections can be safely performed in such patients and it may be an alternative to radiotherapy.
The most common complication in almost all series after lung volume reduction surgery is air leakage. In our series, 34.6% prolonged air leakage was observed and a re-operation decision was taken in 2 patients. On the other hand, cardiac complications such as arrhythmia, myocardial infarction and pulmonary embolism after LVRS are similar to other thoracic surgery operations [24]. Arrhythmia is the most common cardiac complication after LVRS. In the NETT study, approximately 22% of patients developed postoperative arrhythmia requiring advanced medical treatment [25]. Myocardial infarction and pulmonary embolism rates have been reported as 1% and 0.8%, respectively [26]. In our study, the postoperative atrial fibrillation rate was lower than that in the literature. We attribute this to both the low number of our patients and the good evaluation of the patients in the preoperative period.
The most important goal in emphysema surgery is to provide an acceptable improvement in the FEV1 value of patients after lung volume reduction. In our patients, an improvement of 48.3% was detected in the postoperative 6th month measurements compared to the preoperative FEV1 values. This value was found statistically significant in Student’s t test (p < 0.001). This shows that it is possible to provide a good postoperative result after LVRS with appropriate patient selection. A decrease in lung volume with LVRS may improve quality of life with low postoperative mortality and acceptable morbidity. Another important point in these patients is quality postoperative care and respiratory physiotherapy support.
Conclusions
Lung volume reduction surgery increases the quality of life in patients when performed with VATS or bronchoscopically. Which procedure will benefit the patients should be evaluated well in terms of surgical or bronchoscopic preferences due to their different anatomical patterns and concomitant diseases. Therefore, emphysema therapy should be individualized for each patient and managed by a team of experts. LVRS surgery can be preferred safely in patients with low morbidity and mortality values.






