Summary
Balloon aortic valvuloplasty (BAV) is a method of treatment for patients who are temporally ineligible for surgical aortic valve replacement or transcatheter aortic valve implantation. Our aim was to evaluate the efficacy, safety and outcome of therapy in patients treated with balloon aortic valvuloplasty. We retrospectively evaluated the procedural and clinical outcomes of 47 consecutive patients with severe, symptomatic aortic stenosis who underwent balloon aortic valvuloplasty in our center. In conclusion, BAV should be treated as a bridge-to-decision on further treatment. Balloon aortic valvuloplasty has high efficacy and an acceptable adverse events rate. Patients undergoing balloon valvuloplasty are high-risk patients with many comorbidities.
Introduction
Balloon aortic valvuloplasty (BAV) was first implemented in 1985 as a treatment method for patients with severe aortic stenosis, who were disqualified from a surgical procedure due to concomitant diseases. The BAV leads to the extension of aortic cusps and annulus, microfractures of valve calcifications, and a partial separation of the commissures. After the procedure a vast majority of patients report clinical improvement. Nevertheless, it has been confirmed that BAV does not improve the survival rate; usually the stenosis recurs after several months [1–3]. Following transcatheter aortic valve implantation (TAVI) introduction, and as the population ages, a significant rise in the frequency of aortic valvuloplasty is observed, which is not only an integral part of the TAVI procedure, but also a bridging strategy. Balloon aortic valvuloplasty can be also considered as a palliative therapy in individual cases, in which due to severe comorbidities there are contraindications to surgery, and it is impossible to perform the TAVI procedure [4].
Aim
The aim of the study was to evaluate the efficacy, safety and outcome of therapy in patients treated with balloon aortic valvuloplasty and to define clinical characteristics, immediate and distant outcomes of the procedure and factors affecting the 12-month mortality.
Material and methods
We retrospectively evaluated the procedural and clinical outcomes of 47 consecutive patients with severe, symptomatic aortic stenosis (AS) who underwent balloon aortic valvuloplasty in our center. Severe AS was diagnosed on the basis of echocardiographic examination with aortic valve area calculated from the continuity equation less than 1 cm2 (< 0.6 cm2 per 1 m2 of the body surface area). Each patient was analyzed individually, considering their complete clinical picture. All patients were symptomatic with considerably impaired exercise tolerance, reporting stenocardia, with prior syncope or documented ventricular tachycardia. Qualification for the BAV procedure was performed during a Heart Team meeting.
Patients requiring improvement of hemodynamic conditions who at the moment of the decision did not qualify for the surgical treatment or TAVI were qualified for this procedure. The procedure was performed due to two indications: (1) as a bridge therapy before aortic valve replacement (AVR) or TAVI, or as a bridge-to-decision concerning further treatment, (2) as a palliative therapy. BAV was planned as a stand-alone procedure, while coronary angio and percutaneous coronary intervention (PCI) (if needed) were performed prior to valvular intervention.
Balloon aortic valvuloplasty was performed in the cath lab with OR backup by an experienced operator. The transvenous temporary pacing electrode was introduced into the right ventricle in all patients. The decision concerning the application of the Swan-Ganz catheter was left to the discretion of the operator. Puncture of femoral artery was performed using the Seldinger technique and a vascular sheath (9–11 F) was introduced (one diameter larger sheath makes the balloon removal easier and safer). Subsequently, the Amplatz L1 catheter was passed through the aortic valve. The pressure gradient through the aortic valve was measured. Next, after the replacement of the guide wire and the arterial sheath a valvuloplasty balloon (BALTON) was introduced (the size had been selected on the basis of annulus dimensions evaluated in echocardiographic examination; length of the balloon was 40 mm) in the area of the aortic valve. During a rapid stimulation (180/min) repeated inflation of the balloon was performed. According to the operator’s experience a few shorter inflations are more effective in terms of increasing the AVA without additional side effects or the risk of hemodynamic compromise due to prolonged rapid pacing. The result of the procedure was evaluated on the basis of hemodynamic parameters and echocardiographic examination. A procedure in which the aortic valve area calculated from the continuity equation or the Gorlin formula increased by 40% or the mean gradient on the aortic valve decreased by 40% was recognized as an effective one.
The primary endpoints of the study were death and qualification for destination therapy (TAVI or AVR). Secondary endpoints were major complications, defined as peri-procedural death, myocardial infarction, stroke, acute severe aortic regurgitation, need for pacemaker implantation, major vascular complications, life-threatening or major bleeding, acute kidney injury stage 2 and 3, conversion to AVR, classified according to the Valve Academic Research Consortium-2 (VARC-2) criteria [5].
Depending on the patient’s clinical condition, the results of the obtained invasive and non-invasive examinations, and comorbidities, the patient was eventually qualified for conservative treatment, TAVI, or the surgical AVR.
Statistical analysis
Quantitative data are given as means and standard deviations. Qualitative data are displayed as frequencies. The Shapiro-Wilk test was employed to determine whether random samples came from a normal distribution. The χ2 test with Yates’ correction was used to compare categorical variables. The unpaired t-test was used to compare normally distributed continuous variables. One-year survival was estimated with the Kaplan–Meier method and compared with the log-rank test. The effects of the clinical variables on the 1-year mortality were assessed using the multivariate Cox proportional hazard regression models with the results expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). Variables with a significant influence on mortality in univariate analysis were entered into the multivariate model. A p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using the Statistica 7 software package.
Results
The procedure of the percutaneous balloon aortic valvuloplasty was performed in 47 subjects with severe symptomatic aortic stenosis. In the bridge group there were 24 subjects, and in the group of palliative care there were 23 patients.
A vast majority of patients were elderly, with numerous concomitant diseases. A considerable proportion of the group that qualified for the procedure were patients after myocardial infarction, with previous coronary artery bypass graft (CABG) and with neurological disorders.
All patients qualified for the BAV procedure were symptomatic, with 85% of patients having NYHA functional class of III or IV; while syncope appeared in 15% of patients. Baseline clinical characteristics of the study group are presented in Tables I and II.
Table I
Baseline characteristics of patients
Table II
Echocardiographic parameters prior to BAV
[i] EDV – end diastolic volume, ESV – end systolic volume, IVSDd – intraventricular septum diastolic diameter, IVSSd – intraventricular septum systolic diameter, LA – left atrium, LVEDd – left ventricular end-diastolic diameter, LVEF – left ventricular ejection fraction, LVPWDd – left ventricular posterior wall end diastolic diameter, LVPWSd – left ventricular posterior wall end systolic diameter, MR – mitral regurgitation, RV – right ventricle, RVSP – right ventricular systolic pressure, TR – tricuspid regurgitation.
After completion of the balloon valvuloplasty procedure, the maximum gradient on the aortic valve decreased from 92.2 ±31.2 to 61.4 ±22.8 mm Hg (p < 0.001), the mean gradient from 52.2 ±18.2 to 35.5 ±13.4 mm Hg (p = 0.001). The aortic valve area (AVA) increased from 0.54 ±0.18 to 0.8 ±0.22 cm2 (p < 0.001). The increase of AVA by > 40% was achieved in 36 (76%) patients. The left ventricle ejection fraction after the procedure did not change significantly compared to the initial value. Patients with previous severe mitral regurgitations showed more significant clinical improvement after the procedure (mostly reduction of shortness of breath).
Similarly to the echocardiographic analysis, a significant improvement of hemodynamic parameters was observed. Prior to the procedure, the value of the maximum gradient equaled 107.8 mm Hg, and the mean gradient was 54 mm Hg. After the procedure a significant reduction of the maximum gradient to 70.8 mm Hg (p = 0.025) was observed, and the mean gradient decreased to 34 mm Hg (p < 0.001). The aortic valve area increased from 0.61 to 0.93 cm2 (p < 0.0021). The procedure of the percutaneous balloon aortic valvuloplasty was effective according to the assumed criteria in 40 (85.1%) patients.
The BAV procedure was performed with the application of a single balloon with the mean diameter of 21 ±2.29 mm, and with its repeated inflation, performed 3.69 ±1.28 times on average. The procedure lasted 112 ±24 min on average. The radiation dose reached 0.84 ±0.62 Gy and the quantity of the contrast medium was 86.66 ±78.29 ml. In two subjects the procedure was performed with no contrast medium administered (kidney dysfunction, no real need of contrast during BAV, used previously for diagnostic procedures). A vascular sheath with the mean diameter of 9.75 ±1.03 Fr was applied. The sheath was removed with the application of mechanical pressure applied for 15 min.
No death occurred during the periprocedural period. During the first month 3 (6.4%) patients died; two of them had been admitted to hospital in cardiogenic shock. The first patient died on the 24th day after BAV as a result of hemorrhagic complications during surgical aortic valve replacement. The second patient underwent AVR on the 13th day after the balloon valvuloplasty, and died on the 23rd day due to a progressive circulatory failure. The third patient died on the 8th day after BAV as a result of a progressive circulatory failure.
Major complications occurred in 5 (10.6%) patients. Major vascular complications (according to VARC-2) occurred in 4 (8.5%) patients, and minor ones in 4 (8.5%) patients. Most often it was a pseudoaneurysm at the puncture site. A large hematoma requiring transfusion of the packed red cells occurred in 2 patients. In 1 patient a cardiac tamponade occurred, which was treated by a pericardial puncture.
A concomitant myocardial infarction, stroke, and the need of a conversion to a surgical procedure occurred in 1 patient. There were no cases of conduction disorders that would require a cardiac pacemaker insertion. In the post-procedural period deterioration of kidney function was observed in 5 patients, with an increase of creatinine level to 150–200% of the initial value. The percentage of complications is presented in Table III.
Table III
Peri-procedural complications of BAV
| Procedure complications | N (%) |
|---|---|
| Major complications | 5 (10.6) |
| Intra-procedural death | 0 |
| In-hospital death | 3 (6.4) |
| Myocardial infarction | 1* (2.1) |
| Stroke | 1* (2.1) |
| Acute severe AR | 0 |
| Vascular complications: | 8 (17) |
| Major | 4 (8.5) |
| Minor | 4 (8.5) |
| Bleeding: | 7 (14.9) |
| Life-threatening | 1 (2.1) |
| Major | 3 (6.4) |
| Minor | 3 (6.4) |
| Need for pacemaker implantation | 0 |
| AKI stage 1 | 5 |
| AKI stage 2 and 3 | 0 |
| Conversion to AVR | 1* (2.1) |
Destination therapy
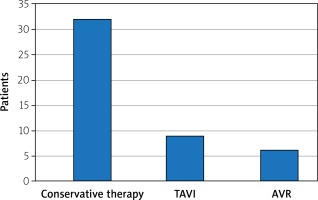
Of all studied patients 31.9% were subjected to the destination therapy (surgical aortic valve replacement or transcatheter aortic valve implantation); TAVI or AVR was applied in 56.5% of patients in the bridge treatment group (the rest of the group was treated conservatively mostly due to lack of consent or concomitant diseases) and 8.35% in the palliative care group.
The destination procedure was performed 91 days after BAV on average (96 days for AVR, 88 days for TAVI).
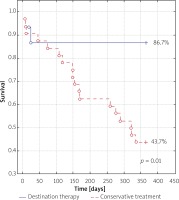
In the group of patients subjected to the destination therapy a considerable reduction of 1-year mortality rate was observed compared to the group of patients who were subjected to the balloon valvuloplasty only (13.3% vs. 56.2% respectively).
The data relating to mortality and the destination therapy are collected in Figures 1 and 2.
Multivariate analysis
The multivariate analysis of the risk of 1-year death revealed that a higher mean gradient on the aortic valve prior to the procedure, female sex, presence of neurological disorders and peripheral arterial disease, were independent factors of 1-year mortality. The procedural success, presence of arterial hypertension (in our opinion it is a statistical bias), and performance of the destination therapy were factors that reduced the mortality in the examined group. The results of the multiple factor analysis are presented in Table IV.
Table IV
Multivariate analysis of the risk of 1-year death
Discussion
Methodology of the procedure
Since the introduction of the BAV procedure in 1985, the equipment used during the procedure has evolved, and a number of improvements have been implemented in the procedure itself. The most important ones include: (1) application of lower-profile balloons allowing for the use of a smaller-diameter vascular sheath; (2) quick simulation technique during inflation of the balloon, enabling one to position it in a precise manner; (3) possibility of using vascular closure devices facilitating the maintenance of haemostasis; (4) improvement of the guide wires applied during the procedure, decreasing the risk of traumatization during the passage through the valve and inside the left ventricle; (5) new imaging techniques, including transoesophageal echocardiography (TEE) and computed tomography (CT), allowing for a more precise evaluation of the dimensions of the ring, the valve, and the anatomic relations. The majority of these contemporary improvements were utilized in the analyzed procedures.
Complications
Over the years a drop in the number of complications connected with BAV procedures has been observed. It is related to the evolution of the instruments, changes in the methodology of the procedure, and the introduction of devices intended for maintenance of vascular haemostasis. The percentage of complications over the first years after the introduction of the BAV procedure equaled 20–25%. According to the latest reports over the recent years it decreased to 6.8–15.6%. Similarly, the percentage of deaths relating to the procedure decreased from 3–5% to 1–2.5% and the percentage of vascular complications from 5–11% to 1.5–7% [2, 6–14].
Over the years, authors of works documenting the effects of valvuloplasty were not using uniform definitions of vascular and hemorrhagic complications; hence a direct comparison of the results is impossible. Uniform definitions of vascular complications were introduced in 2011 on the basis of the works by the Valve Academic Research Consortium [15].
Major complications in our study occurred in 5 (10.6%) patients, which corresponds to the latest reports pertaining to the results of BAV; it is a much smaller percentage than in the studies from the first period after the introduction of the balloon valvuloplasty. None of the patients died in the perioperative period.
In 1 patient from our study there was a release of embolic material (probably from the valve), which resulted in embolization of the coronary artery and a stroke of the central nervous system. An immediate conversion to AVR + CABG was performed in this patient, with a good effect. Major vascular complications occurred in 4 (8.5%) subjects. It is slightly more than in the latest reports. This may result from the fact that vascular closure devices were not applied in the examined group.
The percentage of major complications, such as myocardial infarction, stroke, and conversion to a cardiac surgical procedure was low, which corresponds to the results of other studies [2, 7, 13]. Other major complications including acute aortic regurgitation, ring fracture, or necessity to implant a cardiac pacemaker, did not occur in the examined group. The percentage of their occurrence described in the subject literature was 1–2.6%, 0.3%, and 0.6–4%, respectively [2, 7, 9–14].
Balloon aortic valvuloplasty as a bridge to further treatment
In the examined group BAV constituted a useful tool enabling a bridging therapy and preparation of the patient for the destination therapy in selected cases. After the procedure of aortic valvuloplasty, TAVI was performed in 9 (19.1%) subjects, AVR in 6 (12.8%) subjects, a repeated BAV in one subject. Some patients initially ineligible for the valve replacement procedure or its transcatheter implantation, after the BAV procedure may improve their clinical condition enough to become potential candidates for the destination therapy [10, 16, 17]. Thanks to rapid clinical improvement after balloon valvuloplasty up to 70% of patients can be qualified for surgical treatment or TAVI [18–20]. After the balloon aortic valvuloplasty a clinical assessment and qualification for a further therapy should be performed each time.
In subjects who exhibit a high operational risk and symptoms of unknown origins BAV allows one to predict clinical improvement after TAVI that could be performed. It is important, taking into account the considerable risk and costs connected with the destination procedure. Valvuloplasty can be also performed in patients ineligible for TAVI/AVR due to comorbidities, including antineoplastic treatment. In this group of subjects BAV can bring a temporary improvement while waiting for further treatment [13].
Currently, numerous researchers emphasize the role of BAV as a procedure enabling one to prepare the patient and to qualify the patient for further treatment. In the study carried out by Eltchaninoff et al. Balloon aortic valvuloplasty served as a bridge to AVR/TAVI in 26.3% of patients (AVR 9.6%, TAVI 16.7%) [9], and in the work by Saia et al. in 34.1% (8.6 and 25.5%, respectively) [13]. These are groups comparable with the group described in our study (31.9%). In works which describe the treatment before the introduction of TAVI, BAV served as a bridging therapy to AVR in 27–30% of cases. Considering the fact that the effects of BAV are only temporary, it is crucial that the destination therapy be performed not later than 6–7 months after BAV. In the examined group the time to the destination therapy was shorter: it was 88 days to TAVI and 96 days to BAV.
In the study by Saia et al. 28% of patients initially disqualified from the surgical procedure were subjected to the target treatment thanks to the clinical improvement after the performance of BAV [18]. These results confirm the thesis that careful selection of patients treated by BAV as a bridge-to-decision may have a positive impact on the improvement in the survival of patients.
Study limitations
The present work is a retrospective analysis based on the treatment outcomes from one centre. The number of patients is lower compared to the largest published works in the world. The profile of the treated patients differs from those described in other studies, especially in the ejection fraction, which may affect the results, including mortality. The study does not have any control group.
However, it seems that these limitations do not significantly affect the main message of the study, which is to demonstrate the aim of using percutaneous balloon valvuloplasty in selected patients with severe aortic valve stenosis.
Conclusions
Patients subjected to balloon aortic valvuloplasty are high-risk patients, with numerous aggravating diseases. The procedure of balloon aortic valvuloplasty is characterized by high efficacy and an acceptable level of perioperative complications. The long-term prognosis is good only in patients subjected to the destination therapy. Independent factors that influence the prognosis are a higher mean gradient on the aortic valve prior to the procedure, female sex, presence of neurological disorders, peripheral artery disease, efficacy of the procedure, presence of arterial hypertension, and performance of the target treatment. Unfavorable long-term results of the balloon aortic valvuloplasty speak in favor of the fact that this method should be a bridge to the percutaneous implantation of the aortic valve or its replacement.