Introduction
One of the main causes of death globally is cardiovascular disease (CVD). The World Heart Federation reported that 17.3 million people die from cardiovascular disease per year [1]. By 2030, heart disease and stroke alone will account for more than 23.6 million deaths from cardiovascular disease [2]. Over the next 10 years, there will likely be a further increase in the costs associated with diagnosing and treating cardiovascular diseases due to their increased prevalence. Due to the rise in cardiovascular disease risk factors such as obesity, diabetes, and the aging of the population, there is a significant economic burden linked to cardiovascular disease [3]. Depending on the patient’s severity and risk assessment, various treatments for cardiovascular disease are chosen. Treatment protocols for cardiovascular diseases are primarily focused on improving blood supply and minimizing tissue damage in order to minimize cardiomyocyte loss and increase contractile area. Surgical procedures are typically used to treat severe cases of cardiovascular disease in order to remove blood clots, treat cardiac arrhythmias with artificial pacemakers, and repair the pathological organic changes in the heart. It should come as no surprise that continuing on a regular drug regimen can be challenging; in most situations, treatment must be continued for life [4]. Even though existing therapies have advanced significantly in the last 10 years, non-specific cytotoxicity, poor solubility and absorption, first-pass metabolism, poor biocompatibility, and low bioavailability all contribute to the therapeutic effects of existing cardiovascular drugs being subpar [5].
One of the fastest growing and most active subfields of nanotechnology research, nanomedicine or nanobiotechnology has garnered international interest in recent years. The synthesis methods and properties of various nanomaterials are crucial in the engineering and design of optimal nanocarriers, which in turn affects the shapes, sizes, structures, and delivery functions of nanoparticle-based drug delivery systems (nano-DDS) [6]. Nanoparticles (NPs) are able to precisely deliver drugs to atherosclerotic plaques by taking advantage of their enhanced permeability and retention effect. This leads to better therapeutic effects and less tissue damage [7]. In addition, nano-DDS have significant potential for improving drug efficacy, prolonging drug action, improving bioavailability, passive or active targeting, reducing drug resistance, and reducing adverse drug reactions [8].
Artificial intelligence (AI) and machine learning (ML) have great potential in drug development and bring numerous improvements in various research areas. These include the identification of new targets, a better understanding of diseases and target associations, the selection of drug candidates, protein structure predictions, the design of molecular compounds, improved research into disease mechanisms, and advancing research into prognostic and predictive biomarkers. In addition, AI and ML can provide important inputs for analyzing biometric data through wearable devices, precision medicine, and conducting data analysis for clinical trials to achieve better experimental results in the pandemic era by using appropriate data collection methods and site monitoring [9].
This article reviewed the progress of NPs as drug carriers in the treatment of cardiovascular diseases and how nanoparticles can deliver anti-inflammatory, anti-proliferative, and anticoagulant drugs directly to the surgical site in surgical procedures; improving postoperative outcomes and reducing complications in cardiovascular surgery are also reported. On the other hand, artificial intelligence has been studied for drug delivery.
Nanoparticles
NPs are generally less than 100 nm in size in at least one dimension, and they can be either organic or inorganic structures [10]. While inorganic NPs are made up of a range of microscopic structures such as quantum, mesoporous silicon, graphene, carbon nanotubes, metals, or metal oxides, organic NPs are made up of a variety of biodegradable materials such as lipids, liposomes or micelles, proteins, dendrimers, polymer vesicles, or hyaluronic acid [11]. Metal-organic frameworks, also referred to as porous coordination polymers, are extremely porous and crystalline polymers made up of organic ligands joined by coordinate bonds to metal ions or metal clusters [9]. Polyethylene glycol completely envelops the surfaces of metal-organic framework nanomaterials, reducing the immune system’s clearance [10]. Drug delivery research has focused attention on NPs in recent years due to their unique properties, including easy surface functionalization, tunable porosity, interconnected macropores, and size and shape. Since it leverages circulating cells’ inherent ability to overcome the immunogenicity of nanomaterials, the combination of cell carrier and nano-drug delivery technology is also rapidly gaining popularity [12]. One example of how NPs were used to load rapamycin was by covering themselves in red blood cell or platelet membranes. Biomimetic NPs [13] are known to have a better therapeutic impact in vivo than conventional nano-drug delivery methods [14] and to be able to evade macrophage phagocytosis in vitro [15, 16]. Liposomes, micelles, dendrimers, polymer NPs, and metal NPs are currently the most widely used and comprehensive nanomaterials in the diagnosis and treatment of CVDs.
Dvir et al., 2011 modified ligands to bind selectively to AT1 on liposomes, directing liposomes toward infarcted myocardium [15]. Researchers are becoming increasingly interested in natural nanomaterials that have good intrinsic targeting properties as a result of the ongoing development of nanomedicines [17]. HDL is a widely used endogenous lipid-based NP. It functions by delivering lipids, proteins, or endogenous miRNA to recipient cells in addition to eliminating cholesterol from plaques through reverse cholesterol transport [18].
The lipid-based NPs with a convex lens shape made by Holme et al., 2012 could, when subjected to high blood shear force, release medications into plaques with structural alterations while simultaneously preserving the structural stability of healthy blood vessels [14]. Researchers have improved the efficiency of drug targeted delivery to CVDs by proposing active targeting strategies based on the pathologic features of atherosclerosis, which are based on in-depth studies of specific cells and molecules in the development of atherosclerosis. To precisely deliver a medication to a particular site where it will take effect, active targeting involves manipulating the function of one or more nanocarrier targets. In the early stages of myocardial ischemia-reperfusion injury (IRI), for instance, peripheral blood expression of the angiotensin II type 1 receptor (AT1) is higher.
Methods
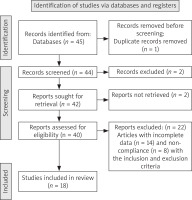
The present study investigated artificial intelligence nanoparticle-based drug delivery systems and targeted delivery of therapeutic for the treatment of cardiovascular diseases by reviewing 45 articles published between 2005 and 2024 with keywords including “Artificial intelligence Nanoparticle”, “Emergency Medicine Unit”, and “Therapeutic for the treatment of cardiovascular diseases” in Scopus, Elsevier, Web of Science and ScienceDirect (Figure 1).
Findings
Application of nanoparticles in the treatment of cardiovascular diseases
Table I shows the application of nanoparticles in the treatment of cardiovascular diseases [18–35]. Traditional NPs still encounter numerous obstacles to efficiently building up in atherosclerotic plaques, including reduced binding efficiency with restricted ligand modification and off-target accumulation due to biomarker expression in normal tissues [19]. Currently, several biomimetic NPs have been created by researchers, such as extracellular vesicle-coated nanoparticles, recombinant HDL NPs, and biomimetic NPs derived from cell membranes. Before medication reaches the plaque, it leaks out through the peripheral blood circulation due to the interaction between lecithin cholesterol acyltransferase and HDL. Moreover, the surface expression of HDL receptors on liver cells tends to hasten the accumulation of HDL in the liver. Therefore, cell membrane and extracellular vesicles have become the most widely used biomimetic strategies in recent years. These biomimetic techniques leverage the biological function and homing capability of cells or their constituent parts to circumvent the immune system, prolong the duration of circulation, and execute customized therapy [20].
Table I
Application of nanoparticles in the treatment of cardiovascular diseases
| Study years | Cardiovascular diseases | Application of nanoparticles and results |
|---|---|---|
| Psarros et al., 2012 [18] | Atherosclerosis | Poly lactic-co-glycolic acid (PLGA), cyclodextrin, and chitosan |
| Katsuki et al., 2014 [19] | When pitavastatin was used alone, there was a significant reduction in plaque rupture caused by PLGA nanoparticles (NPs). | |
| Kim et al., 2020 [20] | Using nanomedicines based on core-shell NPs, the results showed a significant reduction in the amounts of macrophages and cholesterol in plaque following injection, thereby effectively delaying the development of plaque. | |
| Dou et al., 2017 [21] | Comparing drug released from non-responsive NPs to self-assembling acid-sensitive and reactive oxygen species-sensitive non-pro-inflammatory NPs, β-cyclodextrin and encapsulated rapamycin demonstrated superior performance. Both the availability and therapeutic efficacy of drugs released from dual-responsive nanoparticles were improved. | |
| Gao et al., 2018 [22] | Vascular smooth muscle cells (VSMCs) TRPV1 channel was activated by assembling particular antibodies that target transient receptor potential vanilloid-1 (TRPV1) with CuS NPs to form a photothermal switch (CuS-TRPV1). This was done with the aid of a near-infrared laser, which caused Ca2+ to enter the VSMCs and activate the autophagy pathway. Furthermore, CuS-TRPV1 can precisely control TRPV1 channels, which significantly lowers lipid accumulation, and be used in photoacoustic imaging of plaque sites. | |
| Chin et al., 2021 [23] | By targeting the chemokine receptor 2 (CCR2) of synthetic VSMCs, miR-145 was delivered to the plaque site via peptide amphiphilic micelles. This altered the VSMC phenotype and slowed the progression of the plaque. | |
| Alam et al., 2017 [24] | Hypertension | Olmesartan plasma concentrations were 21.8 times higher in the nanoemulsion system, and the drug’s antihypertensive efficacy was improved. Additionally, the drug’s duration of maintenance was extended, and the dosage of olmesartan was reduced by almost three times. |
| Cabrales et al., 2010 [25] | New platform to prepare a nitric oxide (NO) controlled release system | |
| Sun et al., 2017 [26] | Fe3O4 NPs; Cortisol levels in human plasma samples ranged from 1 to 1,000 ng/m, according to results obtained by competitively combining with antibody sites to determine the total amount of cortisol in the body. | |
| Madhurantakam et al., 2018 [27] | To obtain an early diagnosis of hypertension, nanosensors can quickly detect nitric oxide, growth hormone, galectin-3, leptin, sodium ions, and inflammatory factors. | |
| Akagi et al., 2017 [28] | Pulmonary arterial hypertension | In a rat model, beraprost-containing PLGA NPs significantly reduced pulmonary vascular resistance, prevented pulmonary vascular remodeling, and reduced the likelihood of side effects. |
| Pitavastatin, fasudil, and oligonucleotides are a few examples of nanomedicines that have good therapeutic effects in lowering pulmonary artery pressure, prolonging survival times, and blocking pulmonary vascular remodeling. | ||
| Zhu et al., 2016 [29] | Myocardial infarction (MI) | The therapeutic impact of stem cells on MI can be guided and tracked with the help of superparamagnetic iron oxide nanoparticles (NPs) that have special magnetic properties and good biocompatibility. |
| Binsalamah et al., 2011 [30] | Chitosan-alginate nanoparticles were used to target placental growth factor delivery and sustained release for the enhancement of cardiac function at the MI site. | |
| Galagudza et al., 2012 [31] | Silica NPs | |
| Yajima et al., 2019 [32] | In a model of reperfusion injury, ono-1301 NPs increased cardiac blood flow. | |
| Sayed et al., 2020 [33] | Primary rat cardiomyocytes were specifically exposed to miRNA-loaded dendritic polymeric NPs, which effectively stopped reperfusion-induced cardiomyocyte apoptosis. | |
| Vani et al., 2016 [34] | Other cardiovascular diseases | Brain IRI was significantly reduced by fullerene NPs; additionally, brain cells were shielded from oxidative damage by the increased glutathione and superoxide dismutase levels, which effectively scavenged free radicals. |
| Penna et al., 2022 [35] | When utilized as oxygenated nanocarriers, α-cyclodextrin and α-cyclodextrin nanosponges release oxygen continuously and can be perfused in sufficient solution while transporting organs. Enough oxygenation may help the transplanted heart heal after surgery and prolong the useful life of the removed organ. |
Cardiovascular disease patients’ exposure to nano-toxicity
NPs have the ability to cross membrane barriers in the bloodstream and travel throughout the body, affecting organs and tissues at a cellular and molecular level. Nanotoxicity may result from NPs and cells interacting [36]. There was a significant correlation between nanotoxicity and the host immunity, charge, dose, large surface area to mass ratio, surface properties, and narrow size distribution of NPs. Through membrane invasion [37], NPs can enter tissues and cells, where they can be toxic and cause damage to cells. Nanomedicine has garnered significant interest, with its applications in treating cardiovascular diseases and reducing cardiotoxicity being relatively new and expanding. Without causing systemic harm, particularly cardiotoxicity [38], the perfect nanomedicine should be able to target the tissues that make up the plaque, penetrate the core of the plaque, and remove the plaque entirely [39]. Five benefits of nanotechnology exist for lowering cardiotoxicity (Figure 2). To precisely assess the nanotoxicity, long-term comprehensive toxicity assessments are required; additionally, the deleterious effects of nanocarriers on patients remain poorly understood, and more research is needed to determine whether NPs can reduce cardiotoxicity in the treatment of CVDs [4].
Clinical studies on cardiac-specific drug delivery using NPs
MI incidence is especially higher in the event of atherosclerotic plaque-induced thromboembolic events [39]. Shiozaki et al. developed a cholesterol-rich nanoemulsion (NE) to imitate the structure and lipid makeup of low-density lipoprotein (LDL) [40]. As soon as LDL-NE comes into contact with blood, apolipoprotein E is drawn to the area and attaches itself to the LDL receptors on the arterial endothelial cell membrane [41]. Thus, where there are lots of LDL receptors, LDL-NE attaches itself to the atherosclerotic plaque site. The rabbit atherosclerosis model was subjected to paclitaxel delivery via the LDL-NE system, which inhibited the migration of macrophages, the proliferation of smooth muscle cells, and the invasion of the intima via intimal growth [42]. When paclitaxel-LDL-NE was injected intravenously into patients with aortic atherosclerosis between the ages of 69 and 86, half of the patients showed a decrease in the size of their atherosclerotic lesion [40]. This clinical trial was designed to assess the effects of paclitaxel on patients with atherosclerosis. The anti-inflammatory drug methotrexate encapsulated in lipid nanoemulsions (MTX-LDE) is being tested in a new clinical trial on patients with stable coronary disease to determine its safety and effectiveness. Through the acquisition of apolipoprotein, the LDE can attach itself to the atherosclerotic plaque’s LDL receptor. Aortic and coronary CT angiography was used to analyze a number of plaque-related indicators, including biochemical indexes, total lumen value, total atheroma volume, no calcified plaque volume, dense calcified plaque volume, and low attenuation plaque volume. The patients were randomized to receive either an intravenous injection of MTX-LDE or placebo-LDE every seven days for a month. Trial findings support the notion that modifying NPs to include LDL lipid as a ligand for the atherosclerotic plaque is a promising approach for cardiac-targeted drug delivery, even though the study is still ongoing. Contrary to intravenous injection, intracoronary or intramyocardial administration is the delivery method used in many clinical studies [43]. Patients with multivessel coronary artery disease, atherosclerosis, heart failure, and stable angina participated in the NANOM FIM trial. Endoscopic cardiac surgery was used to transfer the patients’ circulating progenitor cells containing silica-gold iron-bearing nanoparticles to atherosclerotic lesions. Patients undergoing NP-based progenitor cell delivery showed a significant reduction in total atheroma volume twelve months following the NPs’ delivery.
Integration of artificial intelligence with nanotechnology
Due to the longer half-lives, higher costs, and lower productivity of recent molecular commodities, pharmaceutics and drug delivery have gained significant attention in the pharmaceutical industry. However, even current formulation development relies on costly, time-consuming, and unpredictable classic trial-and-error experiments. Big data, artificial intelligence, and multiscale modeling approaches are being integrated into pharmaceutics through a new system called “computational pharmaceutics”, which is bringing about a significant potential shift in the drug delivery paradigm. This is due to the exponential growth of computing power and algorithms over the past ten years. Pharmaceutical product development is currently using AI techniques for a number of tasks, such as predicting activity, in vitro drug release, physical stability, in vivo pharmacokinetic parameters, drug distribution, and in vivo–in vitro correlation [44].
When predicting the physical stability of solid dispersion at three and six months in 2011, Binsalamah used machine learning techniques [30]. Gao et al., 2017 investigated the solid dispersion’s dissolving behavior using machine learning [17]. A five-fold cross-validation study yielded 85% accuracy, 86% sensitivity, and 85% specificity in the classification model created by the random forest algorithm to differentiate between the “spring-and-parachute” and “maintain supersaturation” types of dissolution profiles. A regression model with a mean absolute error of 7.78 in 5-fold cross-validation was developed using the random forest algorithm to predict the time-dependent total drug release.
Discussion
The ability of drug delivery to target multiple body receptors and thereby reduce the performance of a particular function is a current issue [38]. Because they can be functionalized to target disease-specific cells, nanocarriers have been found to be advantageous in targeting drugs to specific cells or tissues. This helps to prevent toxicity from being triggered in healthy cells [9]. The size, shape, chemical makeup, and surface characteristics of nanocarriers are some of the characteristics that control how drugs are delivered. Making the ideal nanocarrier DDS is difficult, though [12]. AI and computational methods to assess drug loading, drug retention, and formulation stability can help optimize the nanocarrier–drug compatibility [1]. The efficiency and methodology of experiments in the field of nanotechnology are undergoing significant shifts. Although many laboratories now employ automated systems, the development of AI-based databases and nanocarriers shows great potential for translation. The goal of combining automation and AI suggests the possibility of improving targeted therapeutic nanocarriers for particular patient populations and cell types [2]. The primary areas of interest for molecular modeling studies of DDSs involving nanocarriers are (i) assessing the formation and conformation of the particles, (ii) assessing the delivery and interactions of the particles, (iii) assessing the surface properties of the particles, and (iv) adsorption of the particles on various surfaces [45].
To confirm the characteristics of nanocarriers in vivo, in vitro, and in disease areas, an increasing number of experimental tests are being conducted. High-pressure homogenization, wet ball milling, and anti-solvent sedimentation techniques captured 910 particle size data and 310 PDI data. The performance of high-pressure homogenization and wet ball-milling techniques to produce nanocrystals was demonstrated by the light-GBM models in a satisfactory manner. Furthermore, it is possible to avoid the need for numerous trials with different drug combinations by using affordable theoretical computational techniques. The most popular of these theoretical methods are Monte Carlo simulations and molecular dynamics. Through this approach, quantitative measurements that are challenging to obtain experimentally can be clarified by simulations [39]. Selected nanocarrier scaffolds that are appropriate for a given application are difficult to choose. The desired behavior can also be exhibited by optimizing each nanocarrier. From this perspective, creating a database that aids scientists in determining the right nanocarrier scaffold and its functional groups for a given drug encapsulation and release would be a significant advancement. Under the Collaboratory for Structural Nanobiology [15], efforts have been made to establish a database repository of nanocarriers where scientists can obtain 3D structures and physical and chemical properties. This repository serves as a hub for the explanation, organization, and verification of these structures, much like the Protein Data Bank does. This allows correlations to be made between the structures of nanocarriers and the toxicological, physical, chemical, and biological data associated with them. The Nanomaterial Registry is an additional repository that gathers the body of knowledge about different types of nanocarriers, such as metallic nanocarriers, polymers, and dendrimers. An extensive database dedicated to the safety data of nanomaterials is called e Nano-Mapper.
Nanocarrier and pharmaceutical design and optimization have benefited greatly from the recent advancements in AI technologies. Utilizing a variety of AI techniques effectively has sped up product development, guaranteed product quality, and encouraged productive pharmaceutical research and development. Nevertheless, data loss is a common issue when putting machine learning algorithms into practice [45]. The issue arises from the high expense of pharmaceutical trials and the extended duration of research, preparation, and optimization, as major pharmaceutical companies typically maintain stringent records and data retention policies. In addition, individuals who were previously satisfied with machine learning models’ appropriate performance are no longer satisfied and want to know how they operate. Comprehending machine learning techniques can offer deeper understandings of how pharmaceutical formulations are developed. Research and development prospects in the pharmaceutical sector will increase in the future as AI techniques and the pharmaceutical industry become more integrated [45]. Researchers may also be able to conjugate nanocarriers with different functional groups, because there is still deficient knowledge of nanocarriers in terms of the 3D atomic structure. Researcher evaluation of a suitable scaffold for molecular simulations would be made easier with the help of such a repository. Further investigators who are enthusiastic about managing and interpreting data are desperately needed. Unfortunately, no article was found addressing the use of artificial intelligence for nanoparticle-based drug delivery systems and targeted delivery of therapeutics for the treatment of cardiovascular diseases. It is recommended to conduct studies in this area.
AI in cardiovascular medicine
Over the past few years, a wide range of artificial intelligence and machine learning methods have been effectively investigated in the field of cardiovascular medicine for use in major adverse cardiac and cerebrovascular events, diagnoses, classifications, and mortality predictions.
The potential for better patient care and clinical integration has been shown by these studies. Furthermore, the prospect of natural language processing offering innovative benefits for improved patient outcomes in cardiovascular medicine has arisen due to the flood of data from electronic health records (EHR). Rapid data generation and collection from wearable and other modalities has elevated data-hungry neural network models – a subclass of machine learning – to prominence [41]. Large neural networks, or deep learning, have gained popularity recently in cardiology and have outperformed conventional machine learning [20]. In a similar vein, generative adversarial networks, a cutting-edge and potent technique, have the ability to model data density estimation and produce new examples from its knowledge without labels or prediction ethos. This has significant implications for cardiovascular imaging, including the reduction of noise in low-dose CT scans and the creation of synthetic imaging data. Similar technologies have been developed recently, and it is exciting to read about new research that examines the application of ML in different areas of cardiology [42].
To reduce toxicity, increase efficacy, and achieve target ability of medications, nanocarriers provide innovative platforms. In vitro and in vivo characterization techniques have been introduced by the hundreds of nanocarrier formulations that have been developed over the last few decades. The safety and manufacturing procedures that govern the regulatory approval of those ground-breaking systems have thus become increasingly difficult to standardize. Global market release of formulations based on nanocarriers may be facilitated by certain factors. The administration, biodistribution, metabolism, and elimination of nanoformulations are initial areas of critical toxicity that need to be studied [43]. Second, it is necessary to refine the technical aspects that govern the batch-to-batch reproducibility, characterization, and scalability of nanocarriers. It is imperative that research teams, regulatory agencies, and industry associations collaborate in order to expedite the development of stable, safe, and effective nanocarrier DDS. Artificial intelligence and computational research may never fully replace laboratory testing, but they are vital in speeding up and enhancing target and medication discovery procedures as well as in the ongoing search for new, efficient computational tools for nanoparticle and DDS simulation. Furthermore, the field of data handling and analysis desperately needs more researchers [44].
There are several primary drivers behind the current advancements and research in machine learning and cardiovascular medicine. First, improvements in mobile solutions, EHR, and imaging parameters have made valuable datasets that may capture the heterogeneity of diseases more accessible. Second, in order to integrate high-dimensional data and reveal underlying patterns and perceptions of the diseases or conditions, new and sophisticated learning algorithms and exploratory methods are being developed. Thirdly, cloud computing is becoming more and more popular, offering portable, affordable solutions for advanced data mining and analytics.
The sheer amount and speed of the data allow for quick computational and statistical solutions as well as ML solutions for scalable processes. Still, cardiovascular medicine is not only unwavering in its accuracy and prognostication, but it also tailors the conditions to each patient’s unique situation in order to make precision cardiology a reality. A common platform for genomic data is being used by a number of new initiatives and instruments to promote and streamline precision medicine. In order to extract useful insights from omics data, these tools are developed in partnership with healthcare organizations and businesses. The American Heart Association’s Institute for Precision Cardiovascular Medicine, businesses, and academic institutions are all pursuing and pioneering similar partnerships to apply machine learning to precision cardiology and provide actionable insights. Unquestionably, machine learning and artificial intelligence present opportunities and promise for gaining new insights into cardiovascular diseases and conditions [45].
However, organizational collaborations and shared insights may make processes simpler and intuition and cognizance easier to access. Although the introduction of ML has drastically changed the way conventional research thinking is conducted, promoting a variety of methods for applying ML and exchanging ideas may be able to close a knowledge gap. Classical principles of statistical evaluation need to change in this dynamic age of data explosion and collection. Limiting scientific exploration to predictors derived from statistical uncertainty principles in prediction-driven innovative algorithms is unlikely to improve research. In addition to being strong and active, it should stimulate adaptive cognitive search. The main benefits of AI and ML are insights, risk reduction, and decision support in the pursuit of adaptive reasoning. While it is crucial to use automated reasoning and knowledge representation in cardiovascular medicine, it is also crucial to assess the ethical, legal, and social ramifications and create a set of standards.
Conclusions
Nanocarrier technology for nanomedicine offers distinct benefits and potential for novel concepts, strategies, and techniques in the identification and management of cardiovascular diseases, as well as promising opportunities for medical professionals. When compared to conventional drug delivery techniques, nano-drug delivery directly targets the lesion site by introducing various ligands into corresponding nanocarriers in accordance with various pathological mechanisms and therapeutic approaches. By concentrating the medication in the atherosclerotic plaque region more effectively, this approach enhances myocardial blood flow. However, it is challenging to meet optimal diagnostic and therapeutic expectations due to the fast blood flow, frequent interactions between nanomedicine and various immune cells, blood cells, and biomolecules such as cytokines and chemokines. In nano-DDSs, the biomimetic principle is gaining increasing traction. Biomimetic NPs naturally outperform traditional NPs in terms of evading immune system attack, prolonging in vivo circulation time, and improving targeting. As a result, a successful treatment involves the use of both innovative biomimetic techniques and conventional NPs. Furthermore, the potential of cells as nanocarriers is encouraging due to their potent targeting capabilities, as certain cells possess inherent therapeutic properties. When combined with molecular imaging technology, the above NP design strategy will enable the creation of integrated diagnosis and treatment NPs, which will further yield an abundance of information for the treatment of cardiovascular diseases. In the future, there should undoubtedly be a greater focus on the structural design, targeting, stability, and safety of NPs in order to optimize the therapeutic efficacy of nanomedicine. The components of an ideal NP are as follows: ligands that combine with particular molecules in a selective manner, high-capacity drug-loading nanocarriers, the right drug, and controlled drug release. It is also important to investigate novel targets for efficacious medications, such as ways to use nano-DDSs to encourage myocardial cell regeneration following MI.