Introduction
The basic mechanism in response to stress, especially surgical trauma, is the stimulation of the hypothalamic-pituitary-adrenal axis and the increase in cortisol secretion by the adrenal glands. The plasma concentration of cortisol stays within 40–50 g/dl after a stressful situation [1]. The cortisol level is associated with prognosis – both elevated and low values (characteristic for adrenal insufficiency in response to stress) correlate with a higher mortality rate [2]. The treatment of patients with high values of cortisol has not been fully elucidated, but it is well known that the treatment of adrenal insufficiency improves the survival of patients in a severe condition, including after cardiac surgery [3].
Diagnosing adrenal insufficiency in patients after cardiac surgery is difficult. The systemic inflammatory response syndrome is manifested by low cardiac output, tachycardia and fever – symptoms that we also find in patients with adrenal insufficiency. This diagnosis is more difficult in the older population [4]. Despite numerous studies carried out on the elderly population, the relationship between cortisol secretion and age and sex of patients has not been fully explained [5, 6]. Meanwhile, this issue has important clinical implications. Further research on this topic may help to create standards for treating the older population [5].
Assessment of the adrenal reserve would enable the creation of a treatment standard after cardiac surgery and could improve the prognosis in patients with preoperatively reduced adrenal reserve. Salem’s and Coursin’s groups [7, 8] recommend the use of steroid supplementation in patients with known cortisol secretion disorders, depending on the type of surgery. After cardiac surgery, the minimum dose of hydrocortisone should be between 100 and 150 mg administered for 2 to 3 postoperative days. In patients who are suspected of adrenal insufficiency a short stimulation test is recommended. Patients with suspected or diagnosed adrenal disease subjected to steroid supplementation undergo surgical procedures with better outcomes. The experience of Raja et al. suggests that older patients after cardiac surgery with possible adrenal gland disorders require extended use of hydrocortisone supplementation [9]. The standard has not been developed in this topic.
Aim
The aim of the study was assessment of adrenal reserve and secretion of cortisol in patients over 60 years of age undergoing cardiac surgery.
Material and methods
The study involved 20 consecutive patients of both sexes, aged over 60 who were referred for cardiac surgery. The study was performed at the Department of Cardiac Surgery, Upper-Silesian Heart Center, Medical University of Silesia in Katowice, Poland.
The study was approved by the Institutional Review Board of the Medical University of Silesia, Katowice, Poland (Nr KNW/0022/KB1/74/II/15/16/17). Exclusion criteria were as follows: endocrine disease, therapy with steroids during the 6 months before the surgery, addiction to alcohol or drugs, previous cardiac surgery, diseases of the central nervous system.
After receiving written consent, a short ACTH stimulation test was carried out: blood levels of cortisol were measured at 30 and 60 minutes after administration of 250 μg of Synacthen intramuscularly. Assessment of cortisol secretion was carried out in the morning between 8.00 and 10.00 am – on the day of surgery, on the first day, on the 2nd day and on the fourth day after surgery in blood samples by the electrochemiluminescence method (ECLIA, Elecsys Cortisol II).
The total dose of inotropic drugs (dopamine, norepinephrine, epinephrine) used in every patient was evaluated. Lactate concentration was determined as the mean value of measurements taken every 2 hours during the 1st and 2nd days after surgery.
Statistical analysis
Descriptive statistics were summarized by means and standard deviation as well as frequencies (%) as appropriate. The cross-clamping time, cardiopulmonary bypass time and duration of surgery were presented as median and divided into quartiles. The Shapiro-Wilk test was used for the assessment of normal distribution. The quantitative data were compared with Student’s t-test if normally or log-normally distributed (log-transformed as appropriate). Quantitative data not having a normal distribution were compared by the Mann-Whitney U-test for independent groups. Categorical data were compared between groups with Fisher’s exact test. Qualitative data and quantitative data that did not have a normal distribution for dependent groups were compared by the Wilcoxon pairs order test.
Results
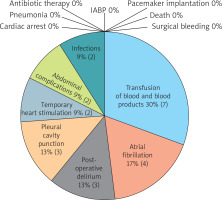
Twenty patients over 60 years of age were included in the study. The group was 85% male. The average age was 69 years. The characteristics of the studied population are presented in Table I. The patients underwent cardiac surgery; 75% of them had coronary artery bypass grafting. The median aortic cross-clamping time was 30.5 (interquartile range – Q1; Q3 – 24; 52) minutes. The median cardiopulmonary bypass time was 58 (45.5; 75.2) minutes. Perioperative data are presented in Table II. There were noted 21 adverse events (Figure 1). The most frequent in the study group was the necessity to transfuse blood and blood products (35%), then atrial fibrillation (20%) (Figure 1).
Table I
Characteristics of study population
[i] BMI – body mass index, BSA – body surface area, EF – ejection fraction, CCS class – Canadian Cardiovascular Society class, NYHA class – New York Heart Association Functional Classification, MI – myocardial infarction, PCI – percutaneous coronary intervention, ACE – angiotensin-converting enzyme, ARBs – angiotensin-II-receptor blockers, CCB – calcium channel blockers, SD – standard deviation.
Table II
Perioperative data
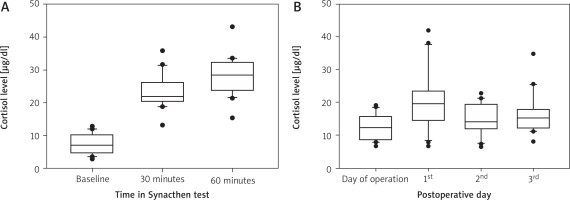
The assessment of the adrenal reserve using the Synacthen test was positive in 19 of the 20 patients enrolled in the study. The mean initial cortisol concentration was 7.5 ±3.1 mg/dl and increased at 30 min after Synacthen injection to 23.3 ±5.0 mg/dl and increased at 60 minutes to 28.0 ±5.8 mg/dl (Figure 2 A). The mean preoperative morning cortisol concentration was 12.4 ±3.95 mg/dl and increased on the first postoperative morning to 21.1 ±9.0 mg/dl (Figure 2 B). The Synacthen test predicted postoperative secretion of cortisol in patients undergoing cardiac surgery (p = 0.04, r = 0.047).
Figure 2
A – Cortisol level during Synacthen test (baseline, 30 minutes and 60 minutes after administration of Synacthen intramuscularly) (mean, standard deviation, each outliers). B – Cortisol level during peri- and postoperative period (mean, standard deviation, each outliers)
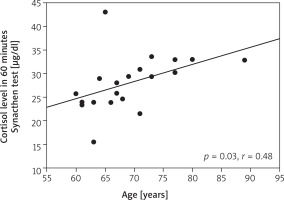
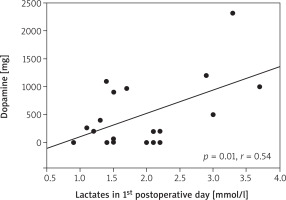
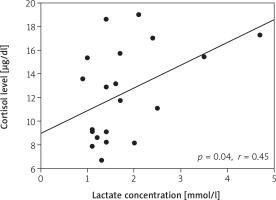
We found an association of cortisol secretion with the age of patients (p = 0.03, r = 0.48) (Figure 3). The concentration of cortisol on the first postoperative day correlated with the total dose of dopamine (p = 0.006, r = 0.58) and adrenaline (p = 0.04, r = 0.47). The concentration of cortisol on the day of surgery correlated with the lactate concentration on day 2 (p = 0.04, r = 0.45) (Figure 4). The concentration of lactates on day 1 correlated with the total dose of dopamine (p = 0.01, r = 0.54) (Figure 5). There was no effect of the type of surgery, cardiopulmonary bypass time or aortic cross-clamping time on the secretion of cortisol in the postoperative period (Table III).
Table III
Correlation between cortisol secretion after operation and duration of surgery, cardiopulmonary bypass time and cross-clamping time
Figure 4
Correlation between cortisol level on the day of surgery and the lactate concentration on the 2nd postoperative day
In our group, there was no strong association between the occurrence of delirium and the cortisol concentration before (p = 0.6, r = 0.13) and after surgery (1st day after operation – p = 0.06, r = 0.42, 2nd day after operation – p = 0.03, r = 0.48, 4th day after operation – p = 0.44, r = 0.18) and results from the Synacthen test (30 minutes after – p = 0.55, r = 0.14, 60 minutes after – p = 0.40, r = 0.2).
Discussion
The cardiopulmonary bypass is an acute stress situation for human organs and tissues. It triggers activation of the hypothalamic-pituitary-adrenal axis (HPA) and induces an increase in cortisol secretion. However, about 10–20% of severely ill patients experience adrenocortical insufficiency [10]. The main symptoms of adrenocortical insufficiency are systemic inflammatory response syndrome (SIRS) and cardiovascular system dysfunctions [10]. However, in the present study, no decreased secretion of cortisol was observed in patients undergoing cardiac surgery in the post-operative period.
The cortisol concentration in patients below 9 mg/dl after administration of 250 mg of corticotropic hormone is called relative adrenal insufficiency (RAI – relative adrenal insufficiency) [11]. It is diagnosed in seriously ill patients and its presence is associated with worse prognosis in sepsis [12]. Relative adrenocortical insufficiency is recognized as inadequate cellular activity of corticosteroid hormones in seriously ill patients. Iribarren et al. diagnosed RAI in 77.5% of the group – the Synacthen test was carried out within 4 hours of the surgery [11]. In our study, such low values of cortisol levels occurred in just one patient. However, no worse course of this patient was observed. In addition, the number of adverse events in the entire study group was low.
Cardiac surgery is a major stimulus that causes endogenous release of catecholamines and stress hormones. The onset of cardiopulmonary bypass increases the concentration of norepinephrine, epinephrine and cortisol in the blood [13]. The Hoda group showed that the cortisol concentration is maintained at an increased level until the end of the surgery, reaching a maximum value within 4–6 hours after the operation. Then, the concentration decreases to the pre-operative values within 24 hours [14]. In the present study, the decrease in cortisol concentration did not occur even on the fourth day after surgery, and the adrenal reserve assessment test was performed before the operation.
Patient anesthesia was performed using propofol and/or anesthetic gases. There are some cardiac surgery studies that confirmed higher blood cortisol levels in patients undergoing propofol anesthesia [15]. This effect was not observed in patients undergoing anesthesia with etomidate. Perhaps higher cortisol levels were maintained due to the way general anesthesia was performed.
In the Iribarren et al. study [11] a higher demand for inotropic drugs was found in the group experiencing relative adrenal insufficiency. This study showed the association of cortisol secretion in the morning after the first postoperative day and the total dose of dopamine and adrenaline. The relationship between lactate concentration on the 2nd day and cortisol concentration on the day of surgery was also demonstrated. It seems that increased secretion of cortisol after cardiac surgery observed in patients with a higher lactate level may be associated with a more difficult postoperative course.
Mu et al. found a relationship between high plasma cortisol concentration on the first day after coronary artery bypass surgery and an increased risk of cognitive impairment in the early postoperative period. Patients with 690 nmol/l had a higher risk of post-operative cognitive impairment (p < 0.001) [16]. We observed a correlation between postoperative delirium and cortisol concentration on the 2nd day after surgery.
The most interesting result was the increase in cortisol secretion with the age of operated patients. Cortisol is a willingly used marker of surgical stress, because its secretion is proportional and positively correlated with the magnitude of surgical stimulation [17, 18]. There is a feedback mechanism between the brain and the secretion of glucocorticoid hormones. Under stress conditions, the brain promotes secretion of cortisol through the corticotropin hormone secreted by the hypothalamus. On the other hand, glucocorticosteroids induce a feedback mechanism by affecting specific receptors in the hypothalamus. In addition, the hippocampus plays an important role in suppressing the pituitary-hypothalamic-adrenal axis in response to stress, at least partially activating the glucocorticosteroid receptors in this region [19]. Glucocorticosteroid receptors in the hypothalamus are gradually lost in the process of normal aging. This results in the weakening of this negative feedback mechanism, which inhibits further secretion of corticosteroids [20]. Numerous studies indicate the existence of a strong correlation between elevated cortisol and hippocampal damage and/or loss of glucocorticoid receptors, both of which are associated with learning disabilities. Loss of glucocorticoid receptors and weakening of the feedback mechanism makes the elderly more prone to chronic hypersecretion of corticosteroids and cognitive impairment after surgery. In patients after non-cardiologic procedures, circadian rhythm disorders have been demonstrated in the cortisol level and are associated with cognitive dysfunction in the early postoperative period [21]. The current study did not assess the cortisol secretion during the day, so it is impossible to identify potential disorders in the circadian rhythm of cortisol secretion.
The main limitation of this study is the small size of the examined group. Nevertheless, the aim of the study was to screen for cortisol secretion disorders in older patients after cardiac surgery.
The study was not randomized. The restrictive inclusion criteria in the study reduced the heterogeneity of the group.
Conclusions
A short Synacthen test allows one to predict secretion of cortisol after cardiac surgery. Greater secretion of cortisol after cardiac surgery could be associated with a more difficult postoperative course. There was no decrease in cortisol secretion with age. The role of cortisol in therapy remains important, and further studies are needed to assess its secretion in elderly patients in cardiac surgery.