Introduction
According to the World Health Organization’s Globocan 2018, with an exact number of a new cases of 20 203, the population age-standardized (world) incidence of breast cancer (BrCa) in Poland is 59.1 per 100 000 [1]. Despite favorable mortality trends since the 1990s in Europe [2], Poland is the only country where the BrCa mortality rate is currently increasing [3]. In 30% of women with BrCa in Poland, the disease progresses to an advanced stage, and in 5–10% of patients it is diagnosed when it has already metastasized to other organs [1]. This requires conducting appropriate surveillance meaning improved BrCa prevention, diagnosis and management in Poland. The main recognized environmental determinants of BrCa are reproductive and hormonal factors, and obesity in postmenopausal women [4]. Genetic factors responsible for the inherited risk of BrCa are of utmost importance for given families carrying common germline mutations in BrCa predisposition genes. Currently 12 out of many other genes are commonly assumed as the most frequent BrCa predisposition genes: ATM,BARD1,BRCA1,BRCA2,CDH1,CHEK2,Nf1,PALB2,PTEN,RAD51C,RAD51D, and TP53 [5]. Regarding the Nf1 gene, the so-called first hit mutation warranted that further carcinogenesis is inherited germinally in patients with neurofibromatosis type 1 (NF-1) [6, 7].
NF-1, with a prevalence of 1 in 2.5–3 thousand live births [6–8] or higher (1 : 1800–2000 [9–11]), is one of the most frequent monogenic trait diseases worldwide, regardless of the race and place of living. The morbidity of NF-1 is impressive as well, due to reported reduction in average sex-independent life expectancy of an 8 to 15-year maximum [12, 13] or even less [9], and the average life longevity of 50–60 years in industrialized countries [12]. The common worldwide observation reveals that a large proportion of NF-1 patients may not be correctly identified or ignored by health practitioners due to inadequacy of health-care systems [6–8]. With 100% penetration of the gene mutation it is characterized by unique, age-dependent extreme variability in phenotypic expression, observed not only between affected patients from different families, but within the same family as well. Age-dependent clinical expressivity of phenotypic manifestations make the clinical diagnosis possible at the 7th–10th year of patients’ life only, but it reaches essentially 100% by adolescence [6–8]. Molecular diagnosis of NF-1 is easily available nowadays but the exact diagnosis is still based mainly on clinical criteria that are highly specific and sensitive for adults with NF-1 (Table 1) [15, 16].
Table 1
NF-1 NIH Consensus Development Conference Criteria of 1988 (NIH-CC-88) (adapted from [7] and [15])
The precise molecular mechanism of NF-1 resulted mostly from pathogenic sequence variants and rarely from microdeletion within the Nf1 gene [17, 18]. This gene encodes neurofibromin, a still enigmatic protein ubiquitously expressed in human cells with varying expressivity depending on the tissue type and developmental stage of the organism [17]. It is a negative regulator of the Ras oncogene. Deregulated Ras expression results in activation of downstream proteins and transcription factors leading to multiorgan disturbances, uncontrolled cell divisions, disturbed apoptosis and many others [6–8, 18–20]. Germline loss and homozygous inactivation of Nf1 lead to tumor formation in NF-1 patients, whereas biallelic somatic loss of Nf1 is commonly found in many different types of cancers worldwide and may indicate that neurofibromin plays a key role in cancer genesis much beyond what is evident in NF-1, a tumor predisposition syndrome [19–21].
The increased risk of BrCa in NF-1 patients (not only women) has been suggested in several cohort and epidemiological studies [22–29]. Somatic mutations of Nf1 seemed to be present in almost 30% of all breast carcinomas regardless of NF-1 diagnosis and have been implicated as genomic drivers in BrCa [30, 31]. The currently published meta-analysis done by Suarez-Kelly suggests that in comparison to the general population the risk of BrCa in NF-1 women of age less than 50 is a five-fold increase and BrCa in NF-1 arises earlier and is more advanced with an increased mortality [29].
As many authors postulated introduction of early BrCa screening guidelines for NF-1 women [22–29], we decided to start with assessment of BrCa risk awareness and current preventive practices among NF-1 women in Poland. According to the cited epidemiological data [3], we may expect that the forthcoming program would improve results of BrCa treatment and mortality in a country where the increased BrCa-related death rate has been growing recently, but significantly in this specific population.
Material and methods
The Coordinated Medical Care Center for Neurofibromatoses and related RASopathies (CMCC-NF/RAS) is one out of 3 reference centers providing integrated and coordinated [7], multispecialty care for patients suffering from NF-1, NF-2, schwannomatosis and Legius syndrome and their mosaic and allelic forms of NF-1 in Poland. The simple and not time consuming, open access and voluntary, NF-1 population-based survey questionnaire was published on the Facebook CMCC-NF/RAS profile (https://www.facebook.com/Neurofibromatozy-strona-dr-Marka-Karwackiego-2319943098240651/). It is selectively dedicated to NF-1 patients from all over the country with 1928 participants and 542 permanently active at the time of publication. It was advertised and spread over as well by the Web Site of the patients’ and parental organization “Alba Julia – Neurofibromatoses-Poland Association” (NGO) (http://alba-julia.pl/) and its FB profile, the only organization for NF patients in Poland. The society assembles more than 1200 NF/RAS patients actually but influences many more. As there is no NF-1 registry in Poland both contacts are the only widely used sources of knowledge and information for NF/RAS patients and their families, concerning medical care official organization and disease course. The questionnaire consisted of 15th closed questions with limited choices was made up utilizing free software offered by “Google Forms: Free Online Surveys for Personal Use” as the resource. The study received approval from the Medical University of Warsaw Ethical Committee.
Results and discussion
The demography and medical characteristics of the responding group of 138 women are presented in Table 2 and Figure 1. Among the responders, whose median age was 34 years, 5 were younger than 16, the official age when a patient has a right to decide by herself in Poland; the youngest one was 13 (Table 2A). In our center the information concerning BrCa risk in NF-1 is usually provided to the patient together with genetic counselling from 16 years of age, if not requested earlier. Anyway, the information is not limited to adults only and all children present in the office during the NF-1 visit may receive the answer to every question they have, modified by the age-specific perception of the patient. As emphasized by the author and ethical committee in the survey preamble, everyone who may feel uncomfortable with information concerning so far undisclosed risk of BrCa always had a chance to receive a precise explanation and counselling. In relation to this (Table 2B.3), the majority of women (67.9%) who finally received subjective information understand properly that the risk of BrCa in NF-1 exceeds the populational risk, but is still not high [27, 29]. In contrast, 5 out of 28 counselled (17.8%) received the information or presumed this risk as high. Unfortunately, 14.3% have been left with this information without an explanation how influential the risk is.
Table 2
Characteristics and demography of the responders (n = 138) of our NF-1 population survey
Fig. 1
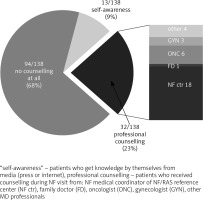
Sources of and general awareness of breast cancer risk among women suffering from NF-1 in our population survey
Excluding the medical specialties not usually engaged in NF-1 diagnosis and care (all together 33%), according to the survey’s responses most frequently NF-1 patients have been diagnosed by so called an NF specialist in NF/RAS centers (18%; Table 2B.1), which may reflect the rising awareness concerning NF/RAS comprehensive care in Poland. The increasing role of NF/RAS Reference Centers, active since 2005 and currently supported by Ministry of Health regulations, ensures the proper early diagnoses and eligibility of NF-1 women for the NF-1 related BrCa prophylactic program [7]. Probably that is why the mean age at diagnosis of NF-1 in the surveyed women as a whole was 16.34 years with a median of 12 (Table 2A). It is promising for the projected program of BrCa prophylaxis in NF-1 women in Poland. Unfortunately in contrast, the oldest patient newly diagnosed as having NF-1 was a 62-year-old woman and the median age of diagnosis in a group of 83 women older than 10 at admission to the professional diagnosis centers (when the diagnosis of NF-1 according to NIH-CC-88 clinical criteria only is easy and unquestionable) was 25. It raises the question of medical care quality in Poland and the possibility to introduce a program of early detection of BrCa in the NF-1 population with success, when a number of them might not be diagnosed properly in the right time.
The important message revealed throughout the survey is significantly limited knowledge about the NF-1-related risk of BrCa (Fig. 1). Thus, 68% of surveyed women did not receive such information at all, whereas only 23% had professional counselling and mostly received in NF centers. There were women as well who found this information in media by themselves (9%) without any support from the professional medical services. Fortunately (Table 2B.2), according to their statements almost half of the group (48.6%) had ultrasound and 11.6% had mammography screening done before the professional counselling concerning NF-1 related risk of BrCa and 35.5% declared self-examination of the breast, whereas only 4.3% disregard screening completely. Breast ultrasound is preferred and the chip method of BrCa screening in NF-1 women but with one limitation – sometimes it is hard to disclose probable BrCa among the multiple neurofibromas affecting both glands. The method of advanced imaging in this situation, preferred for women with NF-1, is still a matter of debate, and European and US standards are conflicting. In the US the chosen method of advanced screening and more precise diagnosis than provided by ultrasound is magnetic resonance imaging [32]. Mammography is assumed as dangerous in NF-1, as irradiation may provoke the malignant transformation of breast plexiform neurofibroma(s) into malignant peripheral nerve sheath tumor [19–21]. In this context, according to the survey results (Table 2B.3), the most frequently advised method of BrCa screening in Poland was breast ultrasound (51.7%) done on a regular yearly basis and MR imaging (25.0%) as a more advanced examination made regularly (not reimbursed by the Polish NHS). Regular mammography has been advised to 14.3% of NF-1 women, which probably reflects the limitations of the Polish health system. The only advice of recommended breast self-examination was given by an oncologist, which is puzzling in regard to the known early risk of BrCa in NF-1 women. What is more important, almost 90% of counselled women declared compliance with the advertised method of BrCa prophylaxis.
There are two final interesting findings, which may reflect the suitability of the early prophylaxis of BrCa in NF-1 women in Poland. The most important is that BrCa has been diagnosed in 4 out of 138 (2.89%; Table 2C) women and before 50 years of age (the most important message). However naïve and simplified, estimation of the expected number of affected women in the surveyed cohort according to BrCa populational incidence in Poland [1] seems to be 0.08 per 138 patients (expected 0.006% vs. actual 2.89%). Thus, the result of the survey is revealing in this respect.
The other important observation is based on the discrepancy between the surveyed cohort demography and the remaining population of NF-1 patients. As shown in Table 1A, in our cohort women with sporadic or spontaneous mutation (71.8% vs. expected app. 50% in general NF-1 population) and nulliparous (or childless – 59.4%, whereas the median age of the group was 34.0 years) dominated the group. This overrepresentation, especially concerning women with spontaneous mutation, may reflect the higher level of fear among NF-1 women who had no experience with the disease which had not burdened their family members in the past. Although it is a pure speculation of the author, probably women growing up in a family where many members were affected and most of them did not present a severe NF-1 phenotype (described in the introduction), might not be scared as much as patients who had not experienced the disease before. It may yet be further confirmed by the relatively high number of NF-1 women undergoing prophylactic breast ultrasound even before the professional counselling. The high proportion of nulliparous women is the finding which may raise a question concerning the fear of NF-1 women against pregnancy and their preferences in regard to high risk of disease transmission. Both observations must be taken into account when a BrCa prophylaxis program is to be established.
Even if the presented cohort is not representative in regard to statistical analyses and limited because of voluntary access and response bias, the represented opinions are very important and provide not only a background for future guidelines preparation, but reveal the ignorance of patients and doctors in Poland concerning the risk and preventive measures of NF-1 related BrCa.
Conclusions
The well-documented risk of BrCa in women suffering from NF-1, confirmed in our study as well, requires implementation of a national program of appropriate surveillance and early detection of BrCa in this given population.
The limited awareness of NF-1 related BrCa risk in Polish patients warrants educational efforts directed both to the NF-1 patients by professional counseling and to the medical community, in order to increase the efficacy of preventive measures and decrease BrCa mortality.








