Introduction
Analgesia in patients with cirrhosis admitted to hospital is an important and challenging management issue. It is complicated by both patient factors and side effects of commonly used medications. In particular, analgesics have the potential to cause significant sedation and constipation, which can in turn precipitate hepatic encephalopathy. Encephalopathy has been associated with increased hospital lengths of stay, admission costs, and life-threatening complications [1-3]. As such, pain management in patients with cirrhosis has generated significant concern among healthcare professionals. As a result, some patients with cirrhosis may be given inadequate doses of analgesia compared to similar patients without liver disease.
The choice of analgesia in patients with cirrhosis is further limited when compared to the general population, with non-steroidal anti-inflammatory medication being relatively contra-indicated due to the risk of upper gastro-intestinal bleeding and worsening renal impairment [4]. Similarly, the use of neuropathic agents such as gabapentin needs to be used with caution, due to concerns regarding worsening renal impairment and over-sedation [5]. Opioids are the most difficult to use with central nervous system depression and constipation occurring even with modest doses; however, fentanyl, tramadol and oxycodone (immediate release) are the opioids of choice within this population [6].
Patients with liver cirrhosis may be denied adequate analgesia or are burdened with recurrent encephalopathy due to incorrect drug choice or dosing. This effect is further compounded by worsening constipation, which is a well-established side effect of opioid analgesia [7] and requires co-existing management with regular aperients such as lactulose.
We performed a prospective cohort pilot study to assess the quality of analgesia in patients with cirrhosis, and monitor their side effect profile in comparison with non-cirrhotic patients. To our knowledge, this is the only prospective study which has evaluated this challenging management issue.
Material and methods
Patients admitted to a tertiary referral hospital with liver cirrhosis who required regular opiate analgesia were prospectively recruited to the study over an 8-month period (group 1). Patients with liver cirrhosis admitted to the ward who did not require opiate analgesia were also recruited as a control group (group 2), as were patients having elective surgery who did not have any evidence of liver disease (group 3).
Baseline data regarding the patients’ demographic characteristics, history of liver disease, and baseline pain were recorded. The aetiology of liver disease, Child-Pugh (CTP) score on admission, and complications of cirrhosis (previous episodes of encephalopathy, ascites, varices and hepatocellular carcinoma) were all collected. Daily records of the type, dose, frequency and route of regular and as required analgesia, and aperients were tabulated. Patients bowel habits were also documented using a nursing bowel chart.
Two surveys were conducted with the participants on a daily basis. These were the Brief Pain Inventory (BPI) (Appendix 1), which was validated for use in non-cancer patients in 2004 [8], and a hepatic encephalopathy assessment tool, the modified orientation log (MO-Log) (Appendix 2), which was first validated for use in cirrhotic patients in 2012 [9].
The study protocol was approved by the Melbourne Health Office for Research and the Human Research Ethics Committee. All patients gave verbal consent.
Procedure
Patients with liver cirrhosis requiring regular analgesia were identified through multiple pathways. The gastroenterology and hepatology, hepatobiliary, colorectal, orthopaedic, plastics, trauma and emergency general surgery wards were contacted on a daily basis to identify new admissions of patients with cirrhosis. Patient histories available at preadmission clinic one day prior to surgery were analysed on a daily basis to identify patients with liver cirrhosis. These patients were then recruited on admission or one day postoperatively.
Ward rounds conducted by the Pain Management Team were attended along with referrals through the Pain Service Nurse to further identify potential patients. The pharmacy department assisted by identifying patients with liver cirrhosis requiring opiate analgesia.
Once patients were identified and verbal consent obtained, the BPI and MO-Log were administered at similar times each day. Each patient’s analgesic regimen, aperient regimen and bowel function were documented on a daily basis. Constipation was defined as no bowel action for 48 hours or longer [10].
Brief Pain Inventory
The BPI is a tool developed by the Pain Research Group at the University of Wisconsin Medical School-Madison to assess clinical pain [8]. The short form, which utilises recall over a 24-hour period, and provides a rating for pain severity and for the degree to which pain interferes with affect and function, was used. Pain severity was assessed by asking the patient to rate their pain at its “worst”, “least”, “on average” and “right now” (scale 0-10). Pain interference with “general activity”, “walking ability”, “work”, “mood”, “enjoyment of life”, “relations with other people” and “sleep” was assessed (scale 0-10).
The first day of pain was day zero for patients who developed pain during their stay. Regular opiate analgesia was defined as requiring more than two doses of opiates for pain control during the admission.
Modified orientation log
The MO-Log is a validated tool for assessing the severity of overt hepatic encephalopathy in hospitalized patients with cirrhosis (scale 0-24) [9].
Statistical analysis
Data analysis was performed using Stata version 13 (StataCorp, College Station, TX). Univariate analysis was used to analyse the quality of analgesia provided for each patient and frequency tables were formulated to compare the baseline characteristics of the three groups. Continuous data variables were expressed as median and range, whereas categorical data were expressed by number of subjects. A p-value less than 0.05 was considered statistically significant.
Pain severity, BPI and MO-Log scores for the three groups were analysed daily for a 6-day period. Comparison between the three groups were analysed using the two-sample Wilcoxon rank-sum (Mann-Whitney) test, Kruskal-Wallis test, and Fisher’s exact test.
Results
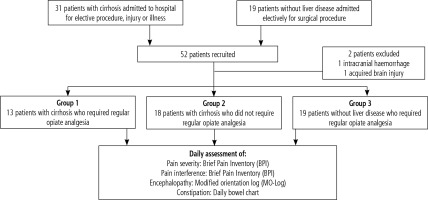
Fifty-two patients were prospectively recruited (Fig. 1). Two were excluded because hepatic encephalopathy could not be adequately assessed (n = 1 intracranial haemorrhage and n = 1 acquired brain injury). Thirty-one had cirrhosis and 19 were non-cirrhotic controls (group 3). Of the 31 patients with cirrhosis, 13 were given regular opiate analgesia (group 1). These 13 patients can be further divided into post-surgical/other procedure (n = 7), or other sources of pain (n = 6).
The clinical characteristics of the three groups are shown in Table 1. The median age of the patients was 52 years, with no significant difference between the three groups. The majority of patients in all three groups were male. The patients with cirrhosis in groups 1 and 2 had a mix of Child-Pugh grade A, B and C, with slightly more Child-Pugh C cirrhotic patients in group 2 (31% vs. 44%, p > 0.05). The etiology of liver disease varied among group 1 and 2, with the commonest being alcoholic liver disease (42%), hepatitis C (29%) and cryptogenic (10%).
Table 1
Clinical characteristics of the study group and control groups
Pain severity, pain interference and MO-Log scores were examined over six days (Table 2). All patients in group 1 and group 3 completed the BPI on at least one occasion. This compares to 8 patients in group B (44%). All patients in the study had at least one MO-Log score recorded.
Table 2
Brief Pain Inventory and modified orientation log scores over the first six days of pain management
Analgesic use differed between groups 1 and 3 (Table 3), with oxycodone being the most commonly used in group 1 (12/13) and oxycodone-naloxone in group 3 (16/19). In group 1, seven patients (54%) received analgesia following surgery or a procedure. The others required analgesia for the following indications: an accidental fall (n = 1), thrombophlebitis (n = 1), septic arthritis (n = 1), and severe abdominal pain (n = 3), two of which were associated with a known hepatocellular carcinoma. The majority of group 3 patients receiving regular opiate analgesia were post-operative or following a procedure (84%).
Table 3
Type of pain, analgesic use and constipation in the three groups
One group 1 patient with Child-Pugh C cirrhosis (CTP score 13) died from progressive liver failure during rehabilitation, 59 days after his orthopaedic procedure.
Pain control
There was no significant difference with regards to pain severity between the 3 groups (Fig. 2A). Group 1 patients complained of pain severity ranging up to 9.8 (Table 2), with the greatest pain on day one, gradually decreasing until day six. Pain severity seemed to settle slower in both group 2 and group 3, with higher scores from day three to six when compared to group 1.
Fig. 2
A) Median pain severity. Depicts the median pain severity as assessed by the Brief Pain Inventory (BPI) over the 6-day study period by participant group. Group 1 – cirrhotic patients who received regular opiate analgesia, group 2 – cirrhotic patients who did not receive regular opiate analgesia, group 3 – patients without cirrhosis who received regular opiate analgesia. B) Median pain interference. Depicts the median pain interference as assessed by the Brief Pain Inventory (BPI) over the 6-day study period by participant group. Group 1 – cirrhotic patients who received regular opiate analgesia, group 2 – cirrhotic patients who did not receive regular opiate analgesia, group 3 – patients without cirrhosis who received regular opiate analgesia. C) Median modified orientation log. Depicts the median modified orientation log (MO-Log) over the 6-day study period by participant group. Group 1 – cirrhotic patients who received regular opiate analgesia, group 2 – cirrhotic patients who did not receive regular opiate analgesia, group 3 – patients without cirrhosis who received regular opiate analgesia. A MO-Log score of ≥ 23 demonstrates normal mentation
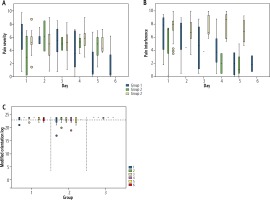
There was, however, a significant difference in pain interference among the three groups on day four (Fig. 2B) [median 3.8 (range 0.5-8.8) vs. 2.0 (0.5-3.6) vs. 8.7 (5.4-10.0), p = 0.02], with cirrhotic patients in both groups 1 and 2 having lower pain interference scores than patients without cirrhosis in group 3. Pain interference followed a similar pattern in groups 1 and 2, with higher scores seen on day one, which gradually decreased over the six days (Fig. 2B). Pain interference remained high for the five days in group 3.
Hepatic encephalopathy
There was no clinically significant difference in MO-Log as all 3 groups maintained a normal median MO-Log score of greater than 23, consistent with normal mentation. However, one group 1 patient with Child-Pugh B cirrhosis (CTP score 8) was severely sedated for 48 hours and required intensive care and intubation. During this time he was given a MO-Log score of 0. For the two days following extubation this patient’s MO-Log scores were 22 and 24. Another group 1 patient with Child-Pugh C cirrhosis (CTP score 11) had reduced mentation, with MO-Log scores of 21 on day one and then 19 and 17 on days three and four. All other group 1 patients maintained a normal MO-Log score of 23 or 24 with no clinical evidence of hepatic encephalopathy despite requiring regular opiate analgesia.
The non-cirrhotic patients (group 3) did not have any significant impairment on MO-Log testing during their hospital stay (median MO-Log score > 23). All group 3 patients scored 23 or 24 throughout the study, with the exception of two patients: one with MO-Log scores of 22 and 23 on days one to four before recovering normal mentation on day five, and another patient with a single MO-Log score of 22 on day one.
All group 2 patients with cirrhosis who did not receive opiate analgesia had a minor reduction in median MO-Log scores (22-24) with the exception of 3 patients, all with Child-Pugh C cirrhosis. One of these patients (CTP score 10) had MO-Log scores of 9 and 6 on days one and two before recovering to 22 on day three, another patient (CTP score 11) had MO-Log scores of 8 and 0 on days one and three, while the third patient (CTP score 10) had a MO-Log score of 17 on day one before recovering to 23 by day three. The fluctuating MO-Log scores in group 2 particularly from days two to six reflected more encephalopathy in the setting of illness.
Constipation
Regular bowel charts were kept for the patients in all 3 groups of the study. Patients in group 3 had a higher incidence of constipation than patients in group 1 or 2 (7.7%, 0.0%, 63.2%; groups 1, 2 and 3 respectively, p < 0.001). This reflects the use of prophylactic aperients in patients with cirrhosis.
Discussion
Hepatic encephalopathy is a feared complication of opiate analgesic use in cirrhotic patients and is associated with significant morbidity and mortality [11]. To our knowledge, no previous studies have tried to prospectively examine the adequacy of analgesia and incidence of reduced mentation in patients with cirrhosis receiving regular opiate analgesia.
Hepatic encephalopathy is a difficult clinical condition to assess as many of the tools are crude and the condition can fluctuate over time [12-15]. In practice, most clinicians rely on the Westhaven Criteria, which are easy to use in a clinical setting but lack sensitivity and specificity [16, 17]. The MO-Log is easy to use, easy to reproduce, and has been validated in a number of studies [9]. It has also been shown to have prognostic value in patients admitted with hepatic encephalopathy or those who develop hepatic encephalopathy during their admission [9]. It was derived from the Orientation log, a 10-part questionnaire used to assess patients with traumatic brain injury [16]. Newer tools such as the EncephalApp Stroop Test which measure the time to do a task on a smart device may provide a reproducible test in future studies [18, 19].
An adequate level of analgesia is an important part of medical care, particularly whilst recuperating from surgery or illness. Adequate analgesia has been shown to facilitate improved patient outcomes in terms of morbidity, early mobilisation and improved quality of life [20, 21]. The choice of analgesics may be slightly different in patients with significant liver impairment, with fentanyl being the drug of choice as it has a short half-life and has minimal hepatic metabolism [6]. However, even longer acting opiates such as oxycodone can be prescribed, provided dosage intervals are adjusted and pre-emptive regular aperients are strictly given.
Our study demonstrated that an effective level of analgesia can be achieved among most patients with advanced cirrhosis without precipitating hepatic encephalopathy. The median MO-Log score was maintained in group 1 patients at a normal level, and in fact these median scores were maintained at a higher level than group 2 patients who did not receive opiate analgesia. This may reflect the impact of inter-current illness on a patient’s cognition or even increased diligence in monitoring for signs of encephalopathy in cirrhotic patients receiving regular opiate analgesia. Interestingly, pain was better controlled in groups 1 and 2 compared to group 3, particularly from day three to six. This may be because analgesia was reduced more rapidly in the patients with fewer comorbidities, or that the cumulative analgesic dose from days one to three was still having an effect on group 1 patients. It is also possible that group 3 patients had more major procedures than group 1 or 2 patients due to a difference in their baseline comorbidities.
There was a lower incidence of constipation in the cirrhotic group receiving opiates compared to non-cirrhotics using opiates, which was presumably due to an increased awareness and regular use of aperients such as lactulose in the cirrhotic patients. These results are similar to previous studies in non-cirrhotic patients, which showed a high incidence of constipation with opioid use [22]. Recently, a bowel function diary has also been validated to assess and monitor opioid-induced constipation, which has been identified as a significant reason why many patients discontinue opioid-based analgesia despite their ongoing pain [23].
Additional strategies to achieve adequate analgesia while preventing hepatic encephalopathy include individualised analgesic plans, increased dosage intervals, reduced doses, use of alternatives, and careful monitoring of mental alertness [24]. These strategies should be implemented with careful consideration not to compromise analgesic effect.
Conclusions
Effective analgesia can be safely achieved in most patients with cirrhosis even in the setting of decompensated liver disease. Further studies to examine the best approach to pain relief in both inpatients and outpatients that assess the effect on cognition are needed to improve our understanding in this area.