Introduction
Developments in surgical techniques have improved the life expectancy of patients with congenital heart disease. Thus, the population who may benefit from long-term follow-up with cross-sectional imaging is increasing [1].
Continued advances in medical imaging have provided the opportunity to diagnose cardiovascular disease using various methods that provide a wide variety of technological requirements, advantages, limitations, and costs. A well-integrated team of professionals collaborating in clinical diagnostic facilities requires sufficient implementation of each method [2, 3]. Despite being the reference diagnostic method, there is a tendency towards the decreased use of diagnostic catheterization, and its use is now predominantly reserved for therapeutic decision-making [4, 5].
Traditionally, cardiologists have relied on echocardiography and conventional angiography to establish the diagnosis of congenital heart disease. Both techniques have potential limitations [6]. The echocardiographic study is operator dependent and limited by an acoustic window, especially in older children and adults [7]. Lung disease further complicates the quality of the echocardiographic image. Conventional angiography is an invasive procedure with its inherent risks, and during angiography, overlapping of pulmonary and systemic circulations may confuse the picture of complex anatomy [8].
Cross-sectional imaging with cardiac magnetic resonance (CMR) or cardiac computed tomography angiography (CCTA) might help overcome the limitations of conventional angiography, such as difficulties in simultaneously depicting the systemic and pulmonary vascular systems and catheter-associated complications [7, 8]. The enhanced pre-operative understanding of congenital heart disease provided by CMR and CCTA makes surgical decision-making straightforward and subsequently might improve outcome [9]. Higher spatial resolution and faster image acquisitions enable CCTA to compete with CMR as the preferred imaging technology for congenital heart disease. The 2 methods may be complementary. The intracardiac anatomy is well depicted by CMR, whereas CCTA provides very clear images of the great vessels [10].
CMR can precisely provide cardiac function and anatomical information that echocardiography and invasive angiography alone cannot [11, 12]. However, the disadvantages of CMR are the necessity for general anaesthesia due to longer duration image acquisition in paediatric patients, is higher cost, limited availability, and image degrading artifacts due to implanted stents and coils [13, 14]. Thailand and other developing countries face the problem of limited resources and availability of cardiac magnetic resonance imaging (MRI). Attributable to the high gantry speed, excellent spatial and temporal resolution, state-of-the-art ultrafast CT scanners have significantly improved the diagnostic performance of CCTA. Ultrafast CCTA can obtain volume acquisition of the entire heart and coronary arteries within 3–4 seconds, with excellent temporal and spatial resolution [15]. The reconstructed images, such as maximal intensity projection (MIP) and 3D volume rendering technique (VRT) images, are of pronounced benefit for the pre-operative preparation and post-operative assessment of congenital heart disease patients. Tachycardia and arrhythmias are the major limitations of CCTA. Nevertheless, these limiting factors have partly been overcome by dual-source CT (DSCT) scanners. Somatom definition Flash (Siemens Healthcare, Forchheim, Germany) is a modern ultra-fast CT scanner that can scan the entire heart in a fraction of the heartbeat, and the total scanning period is 0.25 to 0.27 seconds [16].
CCTA can be considered the imaging modality of choice for certain disorders, such as assessing major aortopulmonary collateral arteries (MAPCAS), pulmonary atresia, and abnormal thoracic vascular structures [6].
Aim
The present study was conducted to accentuate the usefulness of CCTA in assessing congenital cardiovascular diseases across a broad spectrum of pathologic structures.
Material and methods
We studied a total of 78 congenital heart disease patients who had undergone cardiac CTA (CCTA) in our tertiary care academic hospital from January 2017 to October 2018, with complete medical records. Echocardiography is the first-line modality of choice in all patients with congenital heart disease. CCTA imaging was done only in patients for whom echocardiography or angiography was not a completely informative or mandatory confirmation of echocardiography findings. The indication for CCTA was pre-operative evaluation of congenital heart disease patients, which is considered an appropriate indication for CCTA, based on the Society of Cardiovascular computed tomography’s expert consensus document [17]. The CCTA exclusion criteria included kidney dysfunction and a history of allergic reactions to the iodinated contrast agent. The present study was accepted by the Faculty of Medicine Ethics Committee, Khon Kaen University, Khon Kaen, Thailand, research number HE611608.
Dual-source CCTA scanning protocol
Sedation, intubation, and adverse procedural occasions were determined from medical records. Almost all patients could complete CCTA without general anaesthesia. Imaging was accomplished using a second-generation dual-source CT scanner (Somatom Definition; Siemens Healthcare, Forchheim, Germany). The radiation dose is kept to a minimum by appropriately reducing the voltage and tube current. We used 80 kV and 80 mAs, 80 kV and 100 mAs, and 100 kV and 120 mAs, respectively, for children weighing less than 10 kg, 10–19 kg, and 20–30 kg. Automated dose regulation strategies such as CARE dose 4D (Siemens Healthcare) are used to reduce radiation [16]. A 1.5 ml/kg bolus of iodinated contrast medium for a dual-head power injector at a rate of 1.5–2.0 ml/s for a 22-gauge cannula, 3.0 ml/s for a 20-gauge cannula, and 4.0–5.0 ml/s for an 18-gauge cannula, followed by a 10–20 ml saline flush at the same rate as the contrast injection, were administered. A power injector is routinely used to ensure a continuous and regular flow rate. The entire volume of the heart and great vessels was covered in approximately 5 seconds during one breath-hold. Patients were scanned in the supine position [16].
Cardiac CT angiography imaging analysis
All acquired CCTA images were transferred to a dedicated 3D post-processing workstation, and the image analysis was accomplished by an experienced cardiovascular radiologist with 10 years of experience in examining cardiac CTA by using the maximum intensity projections (MIPs), curved multiplanar reformations (cMPRs), and volume rendering technique (VRT). All image data were evaluated using Syngovia software (Siemens Healthcare). MIP and cMPR are mainly used to evaluate curved structures – for example, major aortopulmonary collateral arteries (MAPCAs) and coronary arteries. Minimum-intensity projection (MinIP) is used to evaluate the lung parenchyma and airways. VRT is applied for demonstrated complex anatomy.
Parameters for radiation dose
The imaging sequence, CT dose-volume index (mGy), scan dose-length product (mGy-cm), scan dose-length product (mGy-cm, phantom 16 cm), scan length, and tube current were documented in each study. Individual scans and cumulative procedural dose-length products were documented in mGy-cm.
Radiation dose estimation
To assess the radiation exposure, a procedural dose-length product was used. By calculating the dose-length product with the standard chest conversion factor provided as the scan dose-length product to 0.014, an unadjusted radiation dose in millisievert (mSv) was determined [18, 19]. Conversion factors were further determined as per the recommendations from previous studies [19, 20].
Ethical declarations
This study was reviewed and approved by the local Ethics Committee of Khon Kaen University, Thailand and was registered under reference number HE611608. All methods were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
Continuous variables were described as the median. Analyses were conducted using version 19.0 of SPSS (SPSS Inc., Chicago, Illinois). Furthermore, taking the surgical and/or cardiac catheterization results as the standard reference, the sensitivity, specificity, and diagnostic accuracy of CCTA for cardiovascular abnormalities was appraised.
Results
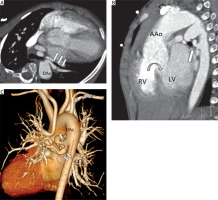
A total of 78 congenital heart disease patients who underwent cardiac computed tomography angiography between January 2017 and October 2018 for pre-operative assessment and had complete medical records were enrolled in the study. Each patient could be classified into more than 1 group because of several findings. The study participants’ ages ranged from 9 days to 74 years (median: 4.5 years). The male: female ratio was 1.16. The number of males was 42 (53.85%) and females 36 (46.15%). Patients’ weights ranged from 2.7 to 68 kg (median: 15.6 kg). The average DLP was 1.41 (0.36–3.28) mSv. There were no procedure-related complications. The results were classified as diagnostic categories (groups 1–5; Tables I–V), and the impact of the procedure on strategizing management was analysed. The common indication for CCTA in the current study was the requirement to evaluate pulmonary artery anatomy and major aortopulmonary collateral arteries (MAPCAs) in the pulmonary atresia with ventricular septal defect patients (n = 11) (Figure 1). Five cases were considered as the confluence of pulmonary arteries. Three cases with confluent pulmonary arteries proceeded to palliative surgical procedure. Of the 6 cases with non-confluent pulmonary arteries, 1 proceeded to unifocalization, and 1 underwent a palliative surgical procedure. Simultaneously, 6 confluent and non-confluent pulmonary arteries had conservative management because their anatomy made surgical intervention excessively difficult. In 3 cases, the growth of pulmonary arteries was observed after a palliative surgical procedure (systemic to pulmonary shunt). The findings are shown in Table I.
Figure 1
A 7-year-old boy with PA with VSD. Cardiac computed tomography angiography axial view (A), sagittal view (B), and volume rendering image (C) demonstrate the major aortopulmonary collateral arteries (MAPCAs) from descending thoracic aorta (white arrows) and revealed ventricular septal defect (B: curved arrow)
PA – pulmonary atresia, VSD – ventricular septal defect, AAo – ascending thoracic aorta, DAo – descending thoracic aorta, RV – right ventricle, LV – left ventricle.
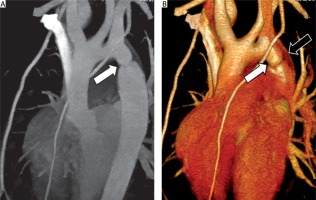
In the group of aortic anomalies (Table II), there were 5 cases of coarctation of the aorta. Three cases were preductal type, and 2 cases were juxtaductal type (Figure 2). Of 5 cases of coarctation of the aorta, 4 proceeded to repair coarctation of the aorta surgically, and 1 case was conservative treatment. There were 2 cases of truncus arteriosus, 1 case of single great artery arising from the heart and bifurcation into the aorta and MPA (Collett and Edwards classification type I), and 1 case of a single great artery arising from the heart and the pulmonary arteries arising separately from the posterior portion of the truncus (Collett and Edwards classification type II), and subtruncal VSD was also found in both cases.
Figure 2
A 10-year-old girl with coarctation of the aorta. Cardiac computed tomography angiography sagittal oblique (A) and volume rendering image (B) shows the abnormal small diameter of aortic isthmus (A and B: white arrows) and demonstrates persistent left SVC (B: black arrow)
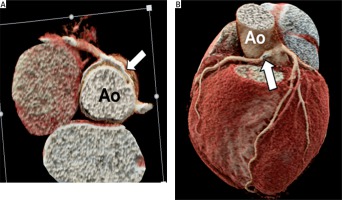
In the group of coronary artery anomalies (Table III), there were 27 cases. Nine of them had anomalous origin of the RCA from the left sinus of Valsalva (Figure 3), of which 3 cases had undergone surgical reimplantation of the coronary artery, and 7 cases had conservative treatment. Five cases had coronary artery fistula, 6 were cases of anomalous RCA with retroaortic course, 4 were cases of single coronary artery, and 2 were cases of anomalous left coronary artery from the pulmonary artery (ALCAPA).
Figure 3
An 8-year-old boy with a right coronary artery from the left sinus of Valsalva, inter-arterial type. Cardiac computed tomography angiography demonstrates the right coronary artery arising from the left sinus of Valsalva and course between aorta and pulmonary artery (inter-arterial type) (A and B: white arrows)
Ao – aorta, PA – pulmonary artery.
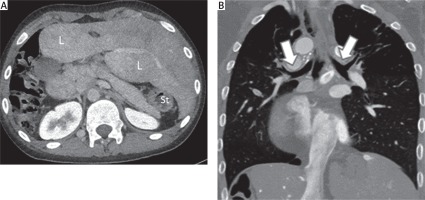
There were 5 cases of heterotaxy syndrome group (Table IV). One case of right isomerism (Figure 4) underwent a central shunt, and another case underwent total surgical correction. Three cases of left isomerism were identified, and 1 of them proceeded to rerouting of the pulmonary vein, 1 case underwent total surgical correction, and another case had conservative treatment.
Figure 4
A 13-year-old boy with right isomerism. Cardiac computed tomography angiography axial (A) and coronal view (B) demonstrate transverse liver (L) and absence of spleen (asplenia) (A) and bilateral eparterial bronchus (B: white arrows)
L – liver, St – stomach.
Miscellaneous group (Table V) includes the following: 19 cases of tetralogy of Fallot (TOF), 1 case of pentalogy of Fallot, 21 cases of atrial septal defect (ASD), 15 cases of ventricular septal defect (VSD), 7 cases of double outlet right ventricle (DORV), and 1 case of patent ductus arteriosus (PDA). Fourteen cases of TOF had total surgical correction or palliative modified Blalock-Taussig shunt. Eight cases of ASD had surgical ASD closure. Five cases of VSD had surgical VSD closure or planning of surgical closure, whereas 10 cases had conservative treatment. All cases of DORV had a palliative surgical procedure or planned surgical correction, and all cases of PDA had undergone PDA closure.
In total, the sensitivity (97.5%; 95% CI: 91.26–99.7%), specificity (100%; 95% CI: 95.32–100%), positive predictive value (100%; 95% CI: 98.7–100%), negative predictive value (99.38%; 95% CI: 97.6–99.84%), and accuracy (98.6%; 95% CI: 96.74–99.99%)) of CCTA, the major findings of which were confirmed by surgical and/or cardiac catheterization, were excellent.
Discussion
The novel generation of CT has transformed the approach to non-invasive evaluation of congenital heart disease. A previous study appraised the application of 3D CT scanning for congenital heart disease, concluding that it had become an invaluable diagnostic and decision-aiding tool, a complement to echocardiography, and often a substitute for invasive diagnostic angiography [21].
The current study attempted to examine the specific advantage of CCTA for a diversity of anatomic abnormalities across the spectrum of congenital heart disease. For the evaluation of pulmonary arteries, CCTA was particularly worthwhile in demonstrating confluence or discontinuity of the pulmonary major aortopulmonary collateral arteries (MAPCAs) (Figure 1). It assisted in the selection of surgical techniques and in evaluating the growth of the pulmonary artery after the procedure. Therefore, CCTA assisted the management decision based (on the regularly questioned of the pulmonary artery anatomy) arteries. For aortic anomalies, CCTA precisely identified the coarctation site, the severity of narrowing, and the associated diseases (Figure 2). CCTA in paediatrics is hindered by high heart rate affecting cardiac motion artifacts. Nevertheless, in selected cases, this limitation did not preclude demonstration of the origin and course of coronary arteries in the present study. CCTA determined the origin and course of the coronary artery precisely in an 8-year-old boy with an anomalous origin of the right coronary artery from the left sinus of Valsalva (Figure 3). Furthermore, CCTA was less invasive than conventional angiography. In particular, CCTA was valuable for visceral heterotaxy, establishing the assorted range of abnormalities in various structures in addition to cardiac abnormalities (Figure 4). Consequently, CCTA assisted in categorizing pulmonary situs, visceral malposition, asplenia, and polysplenia. With the exception of CMR, no other imaging modality demonstrates all the anatomic details of heterotaxy in a single study. The intracardiac anatomy, systemic and pulmonary venous connection, and extracardiac vessels were well defined and validated by surgical findings. CCTA was extremely valuable in illustrating the eparterial or hyparterial position of bronchi in heterotaxy syndrome in the present study (Figure 4), similarly to the previous study, which disclosed the effectiveness of CT for assessing tracheobronchial anomalies in children with congenital heart disease [22].
A major concern in CCTA is radiation exposure. Previous studies revealed that the average radiation exposure per study is diverse, ranging from less than 1 to 28.4 mSv, notwithstanding the short period of scanning [13–16, 23, 24]. The present study tried to minimize radiation exposure by following the as-low-as-reasonably-achievable (ALARA) principle. The average exposure per CCTA study was calculated at 1.41 (0.36–3.28) mSv in the present study compared to natural background radiation for 6 months. A previous study advised that after preliminary valuation with echocardiography, CCTA could possibly substitute invasive diagnostic catheterization for supplementary anatomic clarification in neonates [25]. The current study revealed that this conception could be extended to all age groups for congenital heart disease. Ever since the introduction of CCTA, the number of diagnostic invasive catheterization procedures has decreased dramatically at our institution. Invasive cardiac catheterization has been required only for cases in which physiological information has been essential. CMR does not require ionizing radiation and can provide a range of useful information. Nevertheless, CCTA may be preferable to CMR because of the simplicity of the investigation and the rapidity of image acquisition [21]. Because CCTA has a shorter scan time and has fewer sedation necessities than CMR, it can be accomplished more easily in an unstable patient who needs intensive monitoring and care, and it avoids the associated procedural risks. CCTA has better spatial resolution; consequently, it is superior at identifying the coronary arteries and small collateral vessels. The presence of an implanted pacemaker or defibrillator, other contraindication to CMR, or vascular stents generally cause more artifacts with CMR than CCTA.
There are some limitations of the present study. Firstly, due to retrospective design, missing data were inevitable. Secondly, this study was not intended as a head-to-head comparison of CCTA and other modalities of imaging. We have not attempted to compare CCTA with its major competitor, CMR. Finally, our findings reflect a single-centre experience, and the generalizability of the current results is limited.
Conclusions
Advances in CT scanners and CCTA techniques have resulted in superior image quality with increased use of CCTA for assessing congenital heart disease patients. DSCT with novel technology is the foremost innovation in cardiovascular imaging, with superior temporal resolution and the ability to diminish radiation exposure. The present study has revealed the importance and decision-aiding role of CCTA in assessing congenital heart disease, with excellent diagnostic performance, particularly for answering questions not determined by echocardiography. Anatomical information attained from CCTA might be prudently used to limit the number of views acquired using invasive catheterization, and it might be a substitute diagnostic method to invasive cardiac catheterization, and hence be valuable for planning surgery or interventional cardiac catheterization. Finally, CCTA may then be valuable in terms of reducing global radiation exposure in congenital heart disease patients.






