Summary
We aimed to assess immediate and long-term safety and effectiveness of carotid artery stenting (CAS) using the Roadsaver double-nitinol-layer-micromesh stent in 287 non-consecutive patients. All CAS were performed using the proximal (40%) or distal (60%) neuroprotection system. Clinical and neurological examination as well as duplex ultrasound were completed before CAS, before discharge, at 1, 6 and 12 months, then annually. All CAS procedures were successful. In 30 days major complications (stroke/death) were observed in 9 (3.0%) patients. The 4-year follow-up showed 82% overall survival with no significant difference between asymptomatic and symptomatic patients. The stroke-free survival was 89%. Seven (2.3%) patients developed > 50% in-stent restenosis.
Introduction
Effectiveness and safety of CAS as well as CEA in primary and secondary prevention of stroke have been demonstrated in high-volume cohorts of patients with carotid artery stenosis [1]. The CREST trial in patients with symptomatic and asymptomatic carotid artery stenosis confirmed similar outcomes for CAS and CEA in the primary endpoint (stroke, death, myocardial infarction) but showed relative excess of minor stroke by 30 days – mainly postprocedural – after CAS [2–4]. The risk of embolization as a result of plaque protrusion and releasing debris is greatest after removal of an embolic protection device (EPD) and persists for at least 30 days until full endothelization of the stent [3–6]. It has been shown in several large-scale trials that the risk of releasing debris through the stent struts and distal embolization is significantly higher with open-cell stents as compared to closed-cell stents [3, 7, 8]. On the other hand, proper, individual-based selection of patients and wide use of proximal neuroprotection devices made it possible to achieve a complication rate of 1% if ‘small’ open-cell designed stents are in use for patients with tortuous vessels and non-high-risk lesions [9]. In a search of compromise between open-cell and closed-cell designed stents, novel technology of stent architecture has been developed – by adding an extra micromesh layer to the standard open-cell or close-cell nitinol skeleton. Thus, it was possible to decrease the stent cell area to 0.38 mm2 (Roadsaver, Terumo, Japan) or 0.15mm2 (CGuard, InspireMD, Israel). As this technology is relatively new there have been still concerns about the safety and long-term durability of dual layer micromesh-covered stents (DLMCS) in carotid artery stenting procedures. Some encouraging data on immediate results of DLMCS implantation have already been collected [3, 10].
Aim
Assessment of immediate and long-term safety and efficacy of carotid artery stenting using Roadsaver stent in the treatment of symptomatic or high-risk, extracranial carotid artery stenosis.
Material and methods
This is a retrospective analysis of 298 non-consecutive DLMCS-supported CAS procedures in 287 patients (including 11 patients who underwent bilateral CAS) performed between 2014 and 2019. All treated carotid lesions (> 50% in direct angiography by quantitative angiography – QA) were symptomatic (89, 30%; ipsilateral stoke and/or transient ischemic attack in the last 6 months) or high-risk by morphology (209, 70%; echolucent, highly lipidic, thrombus-containing, ulcerated and “string-sign”) [11]. Most of the patients were men (203, 70.7%) and average age was 70.5 ±8.6 years (range: 51–88). Detailed group characteristics are provided in Table I.
Table I
Baseline characteristics of patients
* Confirmed by angiography > 50% stenosis of at least one coronary artery or history of percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), CABG – coronary artery bypass graft, ICA – internal carotid artery, CCA – common carotid artery, ®out of all 298 CAS procedures, µhigh risk lesion – echolucent, highly lipidic, ulcerated, “string-sign stenoses”, thrombus-containing lesions, ‘soft’ lesions, i.d. with computed tomography density of < 60 HU.
In each patient routinely computed tomography (CT) scan of the head was performed as a part of independent neurological evaluation before carotid intervention. The severity of stenosis (medial peak systolic velocity (PSV) of 3.77 ±1.7 m/s and end diastolic velocity (EDV) of 1.32 ±0.56 m/s) and morphology of carotid plaque were evaluated by duplex Doppler ultrasonography (DUS) and/or CT. The target lesion was subsequently verified by direct quantitative angiography (QA) just before CAS. The angiographic diameter stenosis (DS) was 84.9 ±9.9% (range: 50–99%).
All patients were on high-dose statin and dual antiplatelet therapy (aspirin 75 mg daily and clopidogrel 75 mg daily for at least 3 days before the procedure or loading dose of 300 mg of aspirin and clopidogrel the day before the procedure). During CAS unfractionated heparin (100 IU/kg) was used to achieve activating clotting time in a range of 250–300 s. Atropine (1–2 mg i.v.) was administered just before stent implantation to avoid a severe baroreceptor response. Femoral (93.6%), right radial (5.7% ) or right brachial (0.7%) access was used. The detailed procedural steps including embolic protection device selection and pre/postdilatation balloon sizing have been described previously [11]. The use of an embolic protection device (EPD) was mandatory. In 40.2% of patients proximal and in 59.7% of patients distal embolic neuroprotection was applied. The devices used for CAS are presented in Table II.
Table II
The devices utilized during CAS
Direct stenting was performed in 132 (44.2%) cases whereas in 166 (55.7%) cases predilatation with a small coronary balloon (2.5–3.5 mm) was required. In all cases postdilatation was performed using usually 4.0–7.0 mm diameter balloons to optimize the angiographic outcome. In each patient angiography of intracranial arteries before and comparatively after the procedure was performed. The most representative examples of DLMCS-supported CAS are shown in Figures 1 and 2.
Figure 1
CAS of severe, high risk stenosis of right ICA using Roadsaver stent and Mo.Ma EPD: A – angiography – 90% RICA stenosis, B – Mo.Ma distal balloon inflated in external carotid artery, C – Both balloons of Mo.Ma system inflated, predilatation with 2.5 × 25 mm balloon, D – 8.0 × 25 mm Roadsaver stent implantation, E – postdilatation with 5.0 × 20 mm balloon, F – final angiography showing optimal angiographic result of CAS
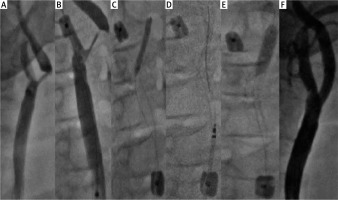
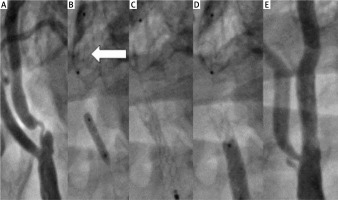
Figure 2
Severe symptomatic lesion of the left ICA and the subsequent steps of CAS using Roadsaver stent and distal EPD-SpiderFX 7 mm, right radial access: A – angiography showing 90% LICA stenosis, B – opening of the filter in the distal segment of LICA (arrow) and predilatation with 3.0 × 15 mm balloon, C – 7.0 × 18 mm Roadsaver stent implantation, D – postdilatation with 5.0 × 20 mm balloon, E – final angiography showing optimal angiographic result of CAS
Patients were discharged 1–2 days after procedure completion with the recommendation of continuation of aspirin, statin (indefinitely) and clopidogrel (for 3 months). In 35 patients with atrial fibrillation vitamin K antagonists or novel oral anticoagulants were restarted after CAS. As the risk of in-stent restenosis is highest in the first year after the initial procedure, clinical and neurological evaluation as well as DUS examination were performed before discharge, after 30 days, 6 months, 12 months and every year afterwards [11, 12].
Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables were expressed as mean and standard deviation (SD). Comparison between baseline and post-procedural results (including follow-up assessments) of %DS, PSV and EDV values were performed using the Wilcox signed-rank test. The log-rank statistic was used to test the differences between symptomatic and asymptomatic subjects. Survival curves were calculated using the Kaplan-Meier method. Two-sided p-values < 0.05 were considered statistically significant. All calculations were done with JMP, Version 14.2.0 (SAS Institute INC., Cary, NC, USA) and using R, Version 3.4.1 (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria, 2017).
Results
The Roadsaver stent was successfully delivered and expanded in all cases. The postprocedural residual stenosis was 0–33% by QA (mean: 11.03 ±9.4%) vs. 84.9 ±9.9% (range: 50–99%) before the procedure (p < 0.001). Control DUS evaluation at 24–48 h after stenting revealed a significant PSV/EDV decrease as compared to initial values (1.3/0.31 ±0.35/0.11 m/s vs. 3.77/1.32 ±1.7/0.56 m/s respectively; p < 0.0001). Mean PSV/EDV values were 1.2/0.34 ±0.58/0.16 m/s at 30 days, 1.2/0.4 ±0.4/0.1 m/s at 6 months, 1.3/0.5 ±0.4/1.7 m/s at 12 months, 1.3/0.4 ±0.4/0.1 m/s at 24 months, 1.29/0.38 ±0.38/0.1 m/s at 36 months and 1.35/0.40 ±0.35/0.10 at 48 months.
During hospitalization one ipsilateral major and three minor (NIHSS < 5 points [12]) ischemic strokes occurred (1.34%), and 1 (0.3%) patient died due to hemorrhagic stroke. Between discharge and 30 days 1 (0.3%) patient died due to hemorrhagic stroke and 3 (1.0%) ipsilateral minor strokes occurred – of those 2 were associated with in-stent thrombosis (0.7%). Thus, the 30-day complication rate was 3.02% (n = 9).
Among patients who died due to hemorrhagic stroke, one case was a consequence of hyperperfusion syndrome (HPS) that occurred 2 days after CAS; the other cause of death has remained unknown and it occurred 21 days after CAS.
Both patients with stent thrombosis were on recommended medications, including dual antiplatelet therapy. The first case of in-stent thrombosis occurred 5 days after CAS in a 76-year-old man, within a 8.0 × 25 mm stent, and led to total artery occlusion and minor stroke. The second thrombosis occurred 14 days after CAS in a 67-year-old man, within a 9.0 × 20 mm stent, and led to subtotal (99%) artery stenosis and minor stroke. Neurological symptoms receded and the thrombus was dissolved within 24 h after introduction of continuous i.v. heparin. Additionally, resistance to clopidogrel was diagnosed and the patient was switched to ticagrelor without further signs of recurrent thrombosis.
One and six months DUS follow-up did not show further in-stent thrombus formation.
Eighteen patients died during 4 years of follow-up (6.3%). Of those, 10 (3.5%) patients died due to cardiovascular complications (myocardial infarction/sudden cardiac death (n = 9), heart failure (n = 1)), and 2 (0.7%) patients died due to ischemic stroke 33 months and 37 months after CAS. In 6 (2.1%) patients the cause of death remains unknown. Excluding in-stent thrombosis cases, in all stroke patients normal in-stent blood flow was assessed by DUS.
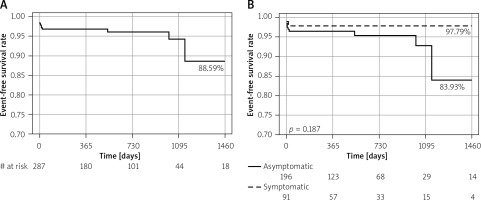
The 4-year follow-up showed 82%/89% overall survival/stroke-free survival with no significant difference between symptomatic and asymptomatic patients (Figures 3 and 4).
Figure 3
Four-year Kaplan-Meier event free-survival curves for patients undergoing carotid artery stenting with Roadsaver stents. A – Overall survival. B – Death-free survival for the symptomatic and asymptomatic patients
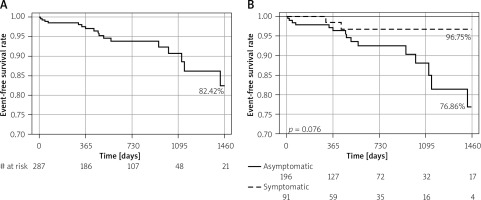
Figure 4
Four-year Kaplan-Meier stroke free-survival curves for patients undergoing carotid artery stenting with Roadsaver stents. A – Stroke-free survival for all patients. B – Stroke-free survival for the symptomatic and asymptomatic patients
Eleven patients could not be contacted; thus, the lost-to-follow-up rate was 3.8%.
Seven (2.3%) cases of in stent restenosis were observed in a period 5–12 months after CAS, 4 patients underwent successful re-angioplasty using drug-eluting balloons (DEB), 2 cases required self-expandable drug-eluting stent implantation, and in one asymptomatic patient re-angioplasty failed due to a problem with vascular access (Leriche syndrome and unsuccessful radial/brachial access attempt). As the patient was asymptomatic he was qualified for optimal medical treatment.
Discussion
Technological development is an on-going process. Despite the fact that the majority of stents implanted in the CREST study population were open-cell with single cell area of 11.5 mm2, the 30-day results were similar to those of the gold standard CEA [3]. As it was previously pointed out, plaque protrusion is an important handicap of first generation stents and may significantly increase the risk of distal embolization. In 2013 a new family of double-layer micromesh-covered stents was introduced. The technology, combining the architecture of open-cell stents (providing optimal flexibility and apposition) with closed cells of micromesh (0.381 mm2, 375–500 µm Roadsaver or 0.15 mm2, 150–180 µm, CGuard) opened the way to perform CAS offering full anatomical restoration of the vessel and optimal protection against plaque protrusion and distal embolization [2, 10]. Indeed, data on optical coherence tomography (OCT) and IVUS confirm Roadsaver stent optimal wall apposition with no significant plaque protrusion through stent cells [2, 3, 13–15]. Moreover, the diffusion-weighed magnetic resonance imaging (DW-MRI) studies showed low, clinically insignificant intracerebral embolization within 24–48 h after CAS and no new spots of embolization within the next 30 days [16, 17]. The very low complication rate of 1% was confirmed by the results of several studies including a total of 550 patients who underwent CAS with implantation of a dual-layer micromesh covered stent (Roadsaver or CGuard) [3, 18–21]. On the other hand, it has been widely discussed that distal embolization may occur at each step of the procedure; thus, the right selection of neuroprotection device is also crucial [11, 22]. Montorsi et al. compared recently the Roadsaver stent with the Carotid Wallstent (single-layer, closed-cell design; Boston Scientific, US) in association with either distal embolic protection (FilterWire EZ, Boston Scientific, US) or proximal embolic protection (Mo.Ma, Medtronic, Italy) in patients with high-risk lipid-rich carotid plaques [23]. CAS performed with the Mo.Ma system gave significantly less microembolic signals in transcranial DUS when combined with the Roadsaver as opposed to the Carotid Wallstent p = 0.043). It is also very important that the clear benefit of using the Mo.Ma device was seen when study population was divided into two groups according to type of neuroprotection use – the Mo.Ma system significantly reduced the mean microembolization signal count during consecutive steps of CAS (lesion crossing, stent crossing, stent deployment and post-dilation) on transcranial DUS.
There have been some concerns raised over in-Roadsaver-stent thrombosis. Yilmaz et al. performed a retrospective analysis of data concerning intracranial interventions in the treatment of acute stroke associated with dual-layer micromesh-covered stent CAS and reported a higher risk of acute thrombosis [24]. Although in the study the Roadsaver stent had a significantly higher rate of acute occlusion as compared with the Carotid Wallstent (p = 0.001), there might have been some imbalance between the study groups, e.g. significantly more patients from the group of single-layer stent received recombinant tissue plasminogen activator before the procedure and a group of patients with acute stent occlusion had a trend to be treated with smaller stent diameters and had a higher platelet count. The authors did not give information about intraprocedural heparin use and not all patients received dual antiplatelet therapy. Another example of acute thrombosis in DLMCS is given by Casana et al., who describe CGuard stent occlusion 4 h after implantation to the extracranial part of the internal carotid artery [22]. We registered two cases (0.7%) of thrombosis in our center – at days 5 and 12 after the procedure. The thrombosis clinically presented as minor strokes. Although it seems that acute in-Roadsaver stent thrombosis is a rare phenomenon, data on long-term follow-up are not yet available. It is important to note that patients from our registry were symptomatic or with high risk lesions [11], with generalized and advanced atherosclerosis. These factors may also influence the risk of periprocedural complications including cerebral embolization. Another important fact is that both ends of the Roadsaver stent are not covered with a dual-layer micromesh layer. If the atherosclerotic plaque is not completely covered by a micromesh part of the stent (e.g. in severe, disseminated atherosclerosis), there is a risk of plaque mobilization and protrusion. Total death/stroke/myocardial infarction in 30-day follow-up in our center was 3.0% in total, 1.2% in symptomatic and 3.8% in asymptomatic (but with high-risk lesions) patients. Although the complication rate for the asymptomatic group slightly exceeds the threshold of 3% accepted by guidelines, it is important to remember that all those patients had high-risk carotid artery stenosis (i.e. echolucent, highly lipidic, ulcerated, “string-sign stenoses”, thrombus-containing, ‘soft’ lesions). High-risk plaque has been shown to be associated directly with high risk of embolization during CAS performed using a conventionally designed stent and distal neuroprotection [25, 26].
It should also be emphasized that the technical success, periprocedural complications and treatment outcomes depend not only on access to innovative technology and proper selection of patients; the learning curve in CAS procedures cannot be neglected [27].
Although it is still an early period of observation, our work has shown that the risk of significant in-Roadsaver-stent restenosis remains in the acceptable range during 4-year follow-up (2.3% as compared to 6.0% reported by CREST investigators [28]). Importantly, it might be presumed that non-mandatory postdilatation in CREST may have influenced the results. However, in comparison to the smallest-cell design Carotid Wallstent postdilated obligatorily with a 4.5–5.5 mm balloon, still the Roadsaver shows much lower prevalence of restenosis [29].
As the Roadsaver low profile makes both femoral and radial/brachial access available there was no need for use of direct carotid hybrid surgical/endovascular access in our cohort. Even so, it has been demonstrated that direct transcervical access for carotid artery stenting is safe and effective, and may be considered in patients with unfavorable anatomy [30].
There are some limitations of our research. It was a non-randomized study in selected patients. No pre- or post-procedural diffusion-weighed magnetic resonance cerebral imaging was performed to determine the true number of cases of cerebral embolization. There was no intravascular imaging assessing the local effect of stent implantation.








