Introduction
The incidence of cutaneous melanoma has been increasing worldwide, resulting in increasing number of melanoma-related deaths in both genders. The highest increase has been observed in the white population [1]. Exposure to ultraviolet (UV) radiation is the main risk factor for developing skin melanoma. While there are many different types of therapies available, the incidence of melanoma increased throughout the period in the world. The rates of melanoma of the skin per 100,000 population increased from 15.2 in 1999 to 22.7 in 2017 in the United States [2]. A sharp increase in incidence rates is also seen in the United Kingdom, with an average annual incidence rate of 9.5/100,000 in 1996–2000 to 25.3/100,000 in 2016–2018 in males and from 12.0/100,00 in 1996–2000 to 24.7/100,000 in 2016–2018 in females [3]. A statistically significant increase in the incidence of melanoma was reported, with an average annual change of 4% in males and 3% in females between 1995 and 2012 in Europe [4]. A similar trend was observed in Lithuania, where the overall melanoma rates increased annually by (APC) 3.9% in males and 2.3% in females; the highest incidence of melanoma was noticed in 60 years and older adult group of both sexes, while the lowest incidence was in a group of 39 years and younger adults [5].
We analysed cutaneous melanoma incidence data from the Lithuanian Cancer Registry 1991–2015 to study the differences of the age, gender, and body-specific sites of skin melanoma.
Material and methods
Ethical approval for this study of population-based cancer registry data was not required. The cancer registry is freely accessible at the National Cancer Institute (Lithuania) website.
The study was based on all cases of primary skin melanoma (International Classification of Disease, Tenth Revision [ICD-10] C43) reported to the Lithuanian Cancer Registry during the period 1991–2015. For the analyses, patients were categorized by sex and melanoma site (head ICD-10 codes – C43.0–C43.4; trunk – C43.5; limbs – C43.6–C43.7; other – C43.8–C43.9). Age-specific and age-standardized incidence rates were calculated. Standardization was performed using the direct method (European standard population). Age-standardized rates (ASR) were calculated for all ages combined and age groups. Corresponding population data by age, sex, and year were available from Statistics Lithuania. Age-adjusted rates by sex and age group were calculated. Adjustment for ASRs was done using the old European standard population. Additionally, the APC was calculated for trends by means of the generalized linear model using Joinpoint software, version 4.5.0.0 (National Cancer Institute, Bethesda, MD, USA). For each of the identified trends, we also fitted a regression line to the natural logarithm of the rates using a calendar year as a regression variable. The 95% confidence intervals for APC were calculated as well. Annual percentage changes were considered statistically significant if p < 0.05. The graphical depiction was implemented using Microsoft Excel software (Microsoft Corporation, Redmond, WA, USA). Ethical review and approval were waived for this study due to population-based cancer registry usage.
Patient consent was waived due to cancer registry data usage. The data that support the findings of this study are available from the corresponding author and at https://www.nvi.lt/naujausi-duomenys/.
Results
According to the Lithuanian Cancer Registry, 6024 new cases of malignant cutaneous melanoma were identified in all age groups between 1991 and 2015 in Lithuania.
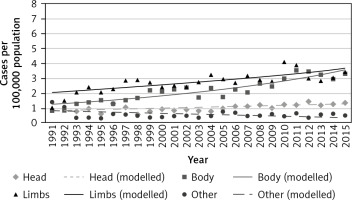
Between 1991 and 2015, the number of new malignant cutaneous melanoma cases increased the most in the trunk and limbs for both sexes. The overall ASR of melanoma in the trunk increased from 0.8 in 1991 to 3.3 in 2015, while in limbs the ASR increased from 1.0 in 1991 to 3.4 in 2015 (Table 1). The highest increase in new cases per 100,000 population (in both sexes) was detected in limbs, and it increased over time (from 2.01 per 100,000 in 1991 to 3.65 per 100,000 in 2015) (Fig. 1).
Table 1
Number of skin melanoma cases and age standardized incidence rates by sex and site in Lithuania, 1991–2015
Malignant cutaneous melanoma was more commonly detected in women. While the anatomic distribution of cutaneous melanomas was the same in 1991, with the highest number of new cases in other sites of the body, it differed in 2015, with 73 cases of melanoma in the trunk in males and 110 cases of melanoma in the limbs in females (Table 1).
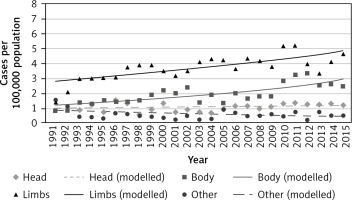
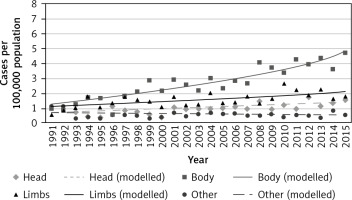
The highest increase in new cases was in limbs with a more than 3-fold increase in ASR from 1991 to 2015 (1.4–4.6) (Table 1). New cases in limbs in women per 100,000 population increased from 2.78 in 1991 to 4.8 in 2015 (Fig. 2). However, the highest increase of site-specific incidence per 100,000 population was found in males. The new melanoma cases in limbs increased from 1.31 in 1991 to 4.68 in 2015 (Fig. 3).
Joinpoint regression analysis of cutaneous melanoma incidence trends identified a statistically significant increase in the incidence of skin melanoma between 1991 and 2015, with the highest APC 5.5 in the trunks of men (95% CI: 5.2–5.9; p < 0.05) and women with APC 3.9 (95% CI: 3.5–4.4, p < 0.05). Since the 1991 Joinpoint, the APC of new cases from melanoma of other/unknown sites of the body decreased in both genders with APC – 1.4 in males (95% CI: –2.1 to –0.7, p < 0.05) and APC – 2.6 in females (95% CI: –3.4 to –1.7, p < 0.05) (Table 2).
Table 2
Results of the Joinpoint Regression Analysis in skin melanoma incidence trends by sex and site, 1991–2015
Discussion
In our study, we found an increase of cutaneous melanoma incidence and mortality in both genders between 1991 and 2015. Skin melanoma of the trunk was more common in males, while the lower or upper extremities were more common among females. The highest mortality annual percent change was observed due to trunk melanoma in both genders.
Overall, the melanoma incidence and mortality rates have been increasing throughout this period throughout the world. It is known that melanoma is about 20 times more common among white people than in people of colour because the epidermis of African Americans (black skin people) has an intrinsic sun-protective factor (SPF) that is much higher than that of white-skinned people [6]. The highest melanoma prevalence was found in Australia, with 37 cases per 100,000 population, while the lowest incidence rate was found in South-Central Asia, with 0.2 cases per 100,000 [7]. This is related to genetic factors, including fair skin, the number of nevi, somatic mutations such as v-raf murine sarcoma viral oncogene homologue B1 (BRAF), neuroblastoma RAS viral oncogene homologue (NRAS), phosphatase and tensin homologue (PTEN), or germline mutations such as cyclin dependent kinase inhibitor 2A (CDKN2A) and the different amount of UV radiation exposure [8]. However, the differences in prevalence can also be related to available prevention strategies, the effectiveness of these strategies, socioeconomic status, and healthcare access [9]. Despite the same availability in the same region, the incidence and mortality rates tend to differ among genders.
Cutaneous melanoma prevalence tends to be higher in women than in men [10]. This can be explained by the complex of factors, such as different metabolism and response to sex hormones, the baseline differences in the immune systems, as well as different mechanisms and mechanics of oxidative stress [10]. In the last decades, the role of sex hormones in melanoma development has been intensively investigated. Melanoma has been shown to express oestrogen-binding receptors. Thus, oestrogens may be associated with early-stage cutaneous melanomas and higher prevalence in females [11]. Exogenous oestrogens as oral contraceptives can also play a role in melanoma pathogenesis [11, 12]. Moreover, it is known that melanoma is the most common cancer associated with pregnancy [13]. However, this oestrogen-related theory is still under investigation, and more studies are needed to confirm this relationship. The second major melanoma risk factor is UVA tanning beds, especially for younger individuals [10]. It is known that early exposure to tanning beds can increase the chance of developing cutaneous melanoma by up to 70–75% [14]. Because females tend to use tanning beds more often than males, the higher prevalence of melanoma in women is strongly associated with it. According to the International Agency for Research on Cancer Working Group on artificial UV light and skin cancer, based on 19 informative studies, ever-use of tanning beds was positively associated with melanoma by a summary of relative risk of 1.15 (CI 95%: 1.00–1.31) [15]. In the European Union, no country has applied a total ban on tanning beds, as was done in Australia. However, the majority of European countries, including Lithuania, have implemented strongly limiting access restrictions for minors such as a strict ban for using tanning beds for persons younger than 18 years [16].
Melanoma risk in the northern hemisphere is related to intermittent or recreational sun exposure and sunburn history [17]. Lithuania restored independence in 1990, and the quality of life and income has been rising since then. The absolute number of trips abroad has been increasing by up to 5% every year and the number of days spent abroad has been rising by 3% every year, and 48% of all travel is recreational [18]. The main destination countries from Lithuania are Turkey, Greece, Egypt, Spain, and Bulgaria, and all 5 countries combined take 56% of all trips abroad. Changes in lifestyle of Lithuanian citizens may be related to the constantly increasing incidence rates of skin melanoma.
The anatomic site of primary cutaneous melanoma lesion is an important prognostic factor. It is known that cutaneous melanoma localized on extremities has a better prognosis compared to melanoma on the trunk, head, and neck. Callender et al. showed that patients with primary skin melanomas of the head or neck and trunk had poorer prognosis in comparison with other anatomical site melanomas [19]. The posterior scalp is considered to be the site with the worst prognosis [20]. In a retrospective cancer registry-based cohort study by Yuan et al., the authors studied United States Surveillance, Epidemiology, and End Results Program data of 262,130 cases, including 152,666 males and 109,464 females of different race (white vs. non-white) [21]. The highest rate of melanoma was found in the trunks of white males with 11.82 cases per 100,000 and in lower and upper extremities of white females with 5.60 cases per 100,000 in upper limbs and 5.98 cases per 100,000 in lower limbs [21]. The highest average APC was in the upper limbs of males with 4.4% and upper limbs and trunk of females with 3.6% in both anatomical sites [21].
Lasithiotakis et al. described data of 1980 patients with invasive cutaneous melanoma diagnosed in Southern Germany [22] and showed the higher incidence of skin melanoma in females (1125 of 1980 cases). The most common anatomic site in males was trunk, while in females it was lower limbs. The same trend was between 1976 and 1989 as well as between 1990 and 2003 [22]. The similar pattern was found in New Zealand by Bulliard in 2000 [23]. The author discovered 6.4 cases per 100,000 person-years in the trunks of males and 10.9 cases per 100,000 person-years in the lower limbs of females [23].
We also observed a similar prevalence difference among genders with higher incidence rates in females. In the Lithuanian population, melanoma was also more commonly diagnosed in the trunk of males (with 4.68 cases per 100,000 in 2015) and limbs of females (with 4.8 cases per 100,000 in 2015). However, the incidence rates were lower in our population.
Despite the higher incidence rates, females have better survival outcomes and lower risk of metastasis than males [24, 25]. This can be related to the lower speed and more local pattern of metastases spread, more frequent medical visits, and earlier detection of the primary tumour [26]. Joosse et al. assessed 11,744 melanoma cases and showed better melanoma-specific survival for females (HR 0.68, 95% CI: 0.56–0.70). A lower risk of disease progression and lower risk of lymph node metastasis as well as visceral metastases were also observed [25]. The National Cancer Intelligence Network observed a similar pattern in their study. The standardized mortality rates (SMR) showed the same variation in mortality between genders. The average SMR for men and women were 3.1 vs. 2.0 per 100,000, respectively [27]. In another study by Bulliard with 3150 death records assessed, the authors found the higher number of deaths in males (1805 vs. 1345). Moreover, the aged-standardized mortality rate was the highest in the trunk in males (ASR 1.62 per 100,000) and in lower limbs in females (ASR 1.23 per 100,000) [23].
Our study has some limitations. Firstly, a true increase may underlie the trend in the incidence and mortality being reported. The fact that both incidence and mortality increased supports a non-artefactual effect. Secondly, the staging is not included. Moreover, comorbidities, drug use, and other cofactors are not included in the analysis. Lastly, we have not assessed cancer-specific vs. other-cause mortality. This information could give a wider view of possible risk factors, relationships to sun exposure, and possible preventative measures.