Summary
Data on the long-term follow-up of patients with myocarditis is scarce. Our study showed that the overall prognosis of patients with myocarditis is good – in terms of both survival and recovery without residual LV dysfunction. Patients with fulminant myocarditis who survive to discharge have a positive prognosis. These findings can be used to reassure patients of the positive prognosis of the disease, and also support the rationale of aggressively managing patients presenting with fulminant myocarditis.
Introduction
Myocarditis is an inflammatory disease of the myocardium. It remains a leading cause of death in athletes and can lead to a debilitating dilated cardiomyopathy [1, 2]. The spectrum of clinical presentation of myocarditis ranges widely from presentation in a subacute form with minimal signs and symptoms to a fulminant form with a rapidly progressive course and hemodynamic compromise [3]. The prognosis of patients with acute/subacute myocarditis varies and is dependent on the clinical presentation, specifically the extent of left ventricular (LV) dysfunction at presentation [4, 5]. There are limited data on the very long-term follow-up of patients with myocarditis. Fulminant myocarditis is a rare, serious form of myocarditis that is often difficult to diagnose. The data on this distinct form of myocarditis are based on relatively small case series with discrepancies regarding the short- and long-term prognosis of these patients [6].
Aim
This study aimed to investigate the long-term clinical course of patients with myocarditis over a 10-year period. It also aimed to identify risk factors for mortality and adverse clinical outcomes. We also aimed to examine the clinical course and outcomes of those with fulminant myocarditis compared to those with non-fulminant myocarditis.
Material and methods
We performed a retrospective analysis of 203 patients diagnosed with myocarditis or perimyocarditis between May 2004 and December 2014 at Rabin Medical Center, Petah Tikva, Israel. Inclusion criteria included patients over the age of 18 years with a diagnosis of myocarditis or perimyocarditis at discharge. Data were collected from the electronic medical chart for each individual patient. The diagnosis of myocarditis was made by the treating physician on the basis of clinical presentation, laboratory values, electrocardiographic changes, and echocardiographic findings, suggestive of myocarditis or perimyocarditis [3, 7]. Echocardiographic assessment was done according to definitions of the American Society of Echocardiography and according to the European Society of Cardiology guidelines on the diagnosis and treatment of acute and chronic heart failure [8–10]. Ejection fraction (EF) was assessed by the modified biplane Simpson’s rule. Echocardiographic LV systolic function was graded according to EF as good (> 60%), preserved (≥ 50%), mildly reduced (41–49%), moderately reduced (30–40%) or severely reduced (< 30%). Regional wall motion abnormalities were graded according to the 17-segment model. Right ventricular (RV) function was assessed by tricuspid annular plane systolic excursion (TAPSE) with TAPSE < 17 mm indicating RV systolic dysfunction and tissue Doppler-derived tricuspid lateral annular systolic velocity (s′) < 9.5 cm/s indicating RV systolic dysfunction. The left atrial (LA) was considered enlarged if the LA volume was more than 34 ml/m2. Pericardial effusion size was graded as small (< 10 mm), moderate (10–20 mm), or large (> 20 mm). Invasive coronary angiography or coronary computed tomography (CT) angiography was also often used to exclude significant coronary artery disease. When cardiac magnetic resonance imaging (CMR) was performed, myocarditis was assessed according to the Lake Louis Consensus Criteria. If 2 of 3 criteria for hyperemia, edema and/or necrosis were present, the CMR was considered indicative of active myocarditis [11]. If deemed necessary, an endomyocardial biopsy was performed and assessed according to the Dallas criteria. These criteria require an inflammatory infiltrate and associated myocyte necrosis or damage not characteristic of an ischemic event for the histopathological diagnosis of myocarditis [12]. The diagnosis of fulminant myocarditis was made on the basis of a clinical presentation of rapidly progressive acute myocarditis with hemodynamic instability. All-cause mortality was confirmed with the records of the Interior Ministry of Israel.
Statistical analysis
Data are presented as mean ± standard deviation for normally distributed variables and as median (interquartile range (IQR)) for non-normally distributed variables. Continuous variables were compared using Student’s t test or the Mann-Whitney test, as appropriate. Categorical variables were compared using χ2 statistics or Fisher’s exact test, as appropriate. In order to analyze the outcomes, we used Kaplan-Meier curves with the log rank test. We adopted a univariate analysis to test the association between presentation characteristics and outcome variables. In order to identify outcome predictors, we employed the Cox proportional hazards regression model for univariate and multivariable analysis. Logistic regression was performed for other variables. Variables in the univariate analysis in which p < 0.2 were included in the multivariable analysis relating to the same variable, together with gender and age. All the tests were two-tailed, a p-value of < 0.05 being regarded as significant. All statistical analyses were performed with SPSS 21. The study was approved by the ethics committee of the Rabin Medical Center and met the requirements of the Declaration of Helsinki.
Results
The study included 203 patients discharged from hospital with a diagnosis of perimyocarditis or myocarditis between May 2004 and December 2014. Patients’ characteristics are presented in Table I. The majority of patients were male (87.7%). The median age at presentation was 33 years (interquartile range: 25.42–38.9). Thirteen (6.4%) patients had a prior history of myocarditis and 85 (41.9%) were current or previous smokers. Most patients had fever (64%) and almost a third (29.6%) had pleuritic chest pain on presentation. The majority of patients (60.1%) presented with a clinical presentation resembling acute coronary syndrome. One hundred and sixteen (57%) patients suffered from a recent upper respiratory tract infection and 34 (16.7%) from a recent episode of acute gastroenteritis. Most patients presented in New York Heart Association (NYHA) Functional Class I. Fifteen patients were diagnosed with fulminant myocarditis, of whom 8 patients presented with an initial presentation of cardiogenic shock. Most patients exhibited electrocardiographic changes at presentation, the most common being ST elevation (60.1%). The majority of patients (79.8%) had normal LV systolic function at presentation and 4.4% had severe LV dysfunction. Twenty-eight percent had a regional wall motion abnormality (RWMA), 12.8% had a pericardial effusion. Seventy-one patients (35%) underwent invasive coronary angiography and 34 (16.7%) coronary CT angiography. Sixty-four (31.5%) patients had a CMR performed, in which most (54) patients had findings consistent with myocarditis according to Lake Louise criteria. The CMR characteristics are presented in Table II. Also in Table II, inflammatory indices were raised amongst the cohort with a median C-reactive protein (CRP) value of 4.93 mg/dl (IQ: 1.79–11.04). Eight patients had an endomyocardial biopsy performed, of which 7 were positive for a diagnosis of myocarditis according to the Dallas criteria and 4 were also positive on the basis of immunohistochemical criteria. Of the 8 patients who had an endomyocardial biopsy, five had presented with fulminant myocarditis. Of these 5, 4 had findings positive for myocarditis on endomyocardial biopsy on the basis of the Dallas criteria for myocarditis, 2 also being positive on the basis of immunohistochemical criteria. None of the biopsies supported giant-cell myocarditis findings. The remaining 3 patients who underwent a biopsy presented with a non-fulminant clinical presentation. One patient had an inflammatory infiltrate with eosinophilia on biopsy and was diagnosed with hyper-eosinophilic syndrome, another had findings consistent with endomyocardial disease with eosinophilia, and the third had findings consistent with non-specific myocarditis.
Table I
Background features and clinical presentation (n = 203)
Table II
Imaging and laboratory features
[i] SD – standard deviation, IQR – interquartile range, Hb – hemoglobin, WBC – white blood cells, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, GOT – glutamate oxaloacetate transaminase, GPT – glutamate pyruvate transaminase, GGT – γ-glutamyl transferase, LDH – lactate dehydrogenase, CK – creatine kinase.
The treatment and outcomes of patients with non-fulminant and fulminant myocarditis are shown in Table III. Most patients with non-fulminant myocarditis were treated with non-steroidal anti-inflammatory agents (NSAIDS) (68.6%) and almost a third with angiotensin-converting-enzyme inhibitors (ACE-I) or β-blockers. Six (40%) patients of those presenting with fulminant myocarditis were treated with corticosteroids. The indication for corticosteroid therapy was hyper-eosinophilic syndrome in 1 patient, systemic lupus erythematosus (SLE) in 1 patient and as empiric therapy in 4 patients. Seven (3.7%) patients with a clinical presentation of non-fulminant myocarditis were treated with corticosteroids. The indications included myocarditis as part of a systemic or autoimmune disease such as SLE (3 patients), eosinophilia (2 patients), chemotherapy-induced myocarditis (1 patient), or due to treatment with corticosteroids during a previous episode of myocarditis (1 patient).
Table III
Treatment and outcomes of patients with non-fulminant and fulminant myocarditis
Immunosuppressive therapy was only used in 1 patient with SLE who presented with fulminant myocarditis. Intravenous immune globulin (IVIG) was also used in 1 patient with fulminant myocarditis. Those with fulminant myocarditis needed more advanced treatment – the majority requiring inotropes (66.7%) and mechanical ventilation (73.3%). Some form of mechanical circulatory support was needed in most patients with fulminant myocarditis – 9 (60%) patients were supported by intra-aortic balloon pump (IABP), 6 (40%) required extracorporeal membrane oxygenation (ECMO) support, and 1 (6.7%) was treated with a left ventricular assist device (LVAD).
The demographic and clinical disparities between the non-fulminant and fulminant myocarditis groups are presented in Table IV. Although most of the patients were male, a larger proportion of women had fulminant myocarditis compared to those with non-fulminant myocarditis (33.3% vs. 10.6%, p = 0.024). No significant age difference was discerned between the two groups. Comorbidities including coronary artery disease, hypertension and dyslipidemia were more prevalent in those with fulminant myocarditis (13.3% vs. 0%, p = 0.005, 33.3% vs. 6.9%, p = 0.005, 46.7% vs. 16%, p = 0.008 respectively). The fulminant myocarditis patients had a significantly longer hospital stay than the non-fulminant group (12.5 days, IQR = 9.5–33 vs. 3 days, IQR = 3–5, p < 0.001). It should be noted that fulminant myocarditis was the first presentation of myocarditis in all the patients who presented with fulminant myocarditis.
Table IV
Demographic and clinical disparities between non-fulminant and fulminant myocarditis
The average follow-up time was 60 ±36.33 months, the overall range being 4.9 to 133.2 months. The overall mortality was 4.4% (9 patients) in the cohort – the fulminant myocarditis group mortality was 26.7% (4 patients) and the non-fulminant myocarditis group mortality was 2.7% (5 patients) (p < 0.001). Two (13.3%) patients in the fulminant group died during hospitalization compared to none in the non-fulminant group (p = 0.005). No statistically significant difference in mortality following hospitalization was found between the two groups (15.4% vs. 2.66%, p = 0.068). The causes of death in those presenting with non-fulminant myocarditis were due to other co-morbid conditions.
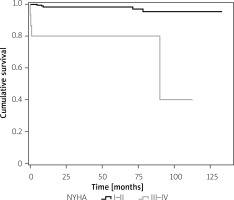
The majority of patients who presented with moderate or severe LV systolic dysfunction improved and returned to normal function or mild dysfunction (14 of 20 patients). No cases of post-discharge arrhythmias were documented. In a multivariable analysis, advanced age was significantly associated with an increased risk of mortality (HR = 1.11, 95% CI: 1.05–1.16, p < 0.001). After adjustment for age and gender, a worsening in NYHA functional class (NYHA III/IV vs. NYHA I/II) (HR = 4.6, 95% CI: 1.18–17.97, p = 0.028), and presentation with fulminant myocarditis (HR = 6.78, 95% CI: 1.72–26.76, p = 0.006) were also associated with a higher risk of mortality. Figure 1 presents the Kaplan-Meier survival curves according to NYHA classes. Higher albumin at presentation (HR = 0.2, 95% CI: 0.07–0.56, p = 0.002) was found to be a positive predictor for survival. After adjustment for age and gender, a lower albumin value at presentation was found to predict a decline in cardiac function (OR = 14.9, 95% CI: 1.24–166, p = 0.033).
Discussion
Our study detailed the clinical presentation and outcomes of 203 patients with myocarditis or perimyocarditis admitted to our institution over a 10-year period. Our main findings showed that most patients were young males with a clinical presentation of non-fulminant myocarditis. There was a low mortality in our cohort and the strongest predictor of mortality was functional level (NYHA classification) at presentation. Of those who had significantly decreased LV systolic function at presentation, most recovered LV systolic function over time. The subgroup of patients presenting with fulminant myocarditis had a more severe course of disease and significantly higher overall and in-hospital mortality.
The clinical manifestations and clinical course of myocarditis can be manifold ranging from mild symptoms to acute heart failure. This study engaged in a longitudinal analysis of 203 patients with non-fulminant and fulminant myocarditis over a 10-year period in order to identity mortality risk factors and other long-term clinical outcomes. Examination of the patient characteristics showed that the overwhelming majority were young men with a recent upper respiratory tract infection. The discrepancies between gender incidence could be due to many potential factors. These include hormonal differences and the potentially protective effect of estrogen [13] as well as a potential underdiagnosis in women. This underdiagnosis could be due to gender disparities in health care utilization or due to atypical clinical presentations. These are factors that have been described in studies evaluating the gender discrepancies in other clinical syndromes such as acute coronary syndromes [14]. Interestingly, the most common comorbidity was smoking, with 41.9% of the cohort being active or previous smokers. The correlation between smoking and an increased extent of late gadolinium enhancement on CMR in those with myocarditis has recently been described [15]. It is hypothesized that an underlying hypoxic smoke cardiomyopathy can potentially make smokers more vulnerable to viral myocarditis [15, 16]. Our findings are consistent with this postulation. In our cohort, the majority of the patients exhibited normal global LV systolic function at the time of presentation, with only 10% presenting with moderate or severe LV dysfunction. As in previous studies, the majority of patients who presented with moderate or severe impaired LV systolic function improved and returned to normal function or only suffered mild impairment [3, 17, 18]. A worrying complication of myocarditis is that of the development of dilated cardiomyopathy, which can occur in up to a quarter of patients [3]. In our cohort, only 5 (2.5%) patients subsequently developed dilated cardiomyopathy, two of whom presented with an initially normal LV systolic function that subsequently deteriorated. After adjustment for age and gender, albumin value at presentation was found to be the only predictor of subsequent impaired heart function (OR = 14.9, 95% CI: 1.24–166, p = 0.033). Lower albumin levels have been found to be associated with an increased risk of mortality in other cardiovascular conditions such as heart failure and myocardial infarction [19, 20]. The potential underlying mechanism of this association could be two-fold. Firstly, albumin is an anti-inflammatory agent and lower levels could be directly related to higher levels of inflammation [21]. Secondly, hypoalbuminemia is associated with an increased risk for the development of heart failure potentially due to the reduced effect of albumin bound drugs and a greater effect on edematous states [19]. This finding supports the use of albumin as a potential prognostic marker in those presenting with myocarditis
The overall mortality was 4.4% (9 patients) in the cohort as a whole, the fulminant group mortality being 26.7% (4 patients) and the non-fulminant group 2.7% (5 patients) (p < 0.001). This finding is similar to other contemporary cohorts [22]. After adjustment for age and gender, the strongest predictor of mortality was NYHA at the time of presentation. This finding is consistent with a prior study in which Kindermann et al. found that NYHA functional class III or IV at baseline was an independent predictor for mortality and the risk of needing a heart transplantation (hazard ratio, 3.20, 95% confidence interval: 1.36 to 7.57, p = 0.008) [5]. In our study, presentation with fulminant myocarditis and a low albumin level were also associated with a higher level of mortality.
The incidence of fulminant myocarditis was 7.4% in our cohort, resembling other series [23]. There was a significantly higher percentage of women in the fulminant group than the non-fulminant group (33.3% vs. 10.6%, p = 0.024). This finding is supportive of other studies in which female gender has been associated with a higher risk of in-hospital mortality and myocarditis related complications [24, 25]. While the mortality rate in the fulminant group was significantly higher than that in the non-fulminant group, the disparity was due to the higher mortality amongst fulminant myocarditis patients during the hospitalization itself (13.3% vs. 0%, p = 0.005). These findings support the rationale of aggressively managing patients presenting with fulminant myocarditis in the acute phase, as those who recover have a good long-term prognosis. This is compared to those with non-fulminant myocarditis, who are less ill in the first place. Early, small studies supported the notion that the prognosis of patients with fulminant myocarditis who survived hospitalization is similar to that of non-fulminant myocarditis patients [23, 26]. However, this is not supported by the findings of a more recent study by Ammirati et al., where LV function of those with fulminant myocarditis remained lower than their counterparts at long-term follow-up [22]. Further studies on long-term follow-up of these patients are needed, especially in the era of higher availability of mechanical circulatory support.
The strength of our study is that it concerns a relatively large cohort of all-comer patients, with a long follow-up of up to 10 years, with most patients being followed up for up to 4.6 years. Our study had various limitations. It was single-center and retrospective. Our cohort extends from 2004. At that time, imaging and endomyocardial biopsies were rarely done or available – thus a lot of the early data are based on patients with clinically suspected myocarditis. Events were identified through the patients’ electronic medical record and summaries of outpatients’ clinic visits. Data were almost complete in terms of mortality but the cause of death for 1 patient was missing. Some patients did not return for follow-up at our institution. While CMR has an important diagnostic role in cases of suspected myocarditis [3], only about a third of the patients in our cohort underwent a CMR test as CMR only became available at our institution in 2008 and as such was unavailable in the early years of this study. Due to only late uptake of this investigative tool at our institution, we could not explore the association between the CMR findings and patient outcomes. Previous studies have found that late gadolinium enhancement burden and genetic predisposition are associated with an increased risk of dilated cardiomyopathy, however, in our cohort only a minority of patients underwent a CMR and none had genetic testing [4, 5, 27]. Additionally, family history of cardiomyopathy was not available to us. In our cohort, an endomyocardial biopsy was only performed on 8 patients. Endomyocardial biopsies are not standard procedure in our center for assessing patients with suspected cardiomyopathy or acute myocarditis as it is an invasive procedure with a potential of false-negative sampling. It was performed in those patients for whom the information derived from the endomyocardial biopsy was deemed to be vital to the management strategy and in whom performing an endomyocardial biopsy was viable. Due to this small number, we could not explore the association between the endomyocardial biopsy findings and patient outcomes. The AHA/ACCF/ESC scientific statement on the role of endomyocardial biopsy in the management of cardiovascular disease acknowledges that endomyocardial biopsies are not routine practice and give high levels of recommendation to perform endomyocardial biopsies in life-threatening clinical presentation [28]. Furthermore, our findings are based on the Dallas histopathological criteria and immunohistochemistry, and did not include viral genome analysis.
Conclusions
Our study showed that the overall prognosis of patients with myocarditis is good – in terms of both survival and recovery without residual LV dysfunction. Patients presenting with fulminant myocarditis have a higher rate of in-hospital mortality compared to those with non-fulminant myocarditis, however, those surviving the hospitalization have excellent long-term outcomes. The strongest predictor of mortality was functional level (NYHA classification) at presentation.