Summary
Coronary chronic total occlusions (CTO) are increasingly encountered during invasive and non-invasive coronary angiography and remain the most challenging lesions for percutaneous coronary intervention (PCI). During recent years success rates and safety outcomes of CTO PCI have substantially improved, and successful recanalization of CTO has been shown to relief angina and improve the quality of life based on randomized clinical trial data. Nevertheless, and contrary to the Western European countries, CTO PCI is significantly underused and accounts for < 3% of all PCI in Poland. The current expert consensus summarizes the rationale, clinical outcomes as well as technical and safety issues of CTO PCI. Significantly, we have presented the modified hybrid algorithm (the “so called” Polish hybrid algorithm) providing some unique refinements to the contemporary CTO PCI strategies. Finally, the list of succinct and practical recommendations on CTO PCI has been provided. Continuous efforts are urgently needed to expand the knowledge on CTO PCI and close the gap between Poland and Western European countries.
Introduction
During recent years success rates and safety of percutaneous coronary intervention (PCI) in chronic total occlusion (CTO) have substantially improved, particularly due to the introduction of new techniques and dedicated equipment as well as specialized training programs of CTO operators. Development of new wires and microcatheters, refinement of retrograde and hybrid techniques as well as periprocedural guidance using invasive and non-invasive imaging tools allowed to achieve success rates exceeding 90% in luminary centers with highly experienced CTO operators [1–3]. The current consensus document summarizes contemporary data on CTO PCI including up-to-date recommendations of the EuroCTO Club, Global Consensus and Polish experts opinions to help disseminate the latest knowledge and recommendations among practicing physicians [4, 5].
Coronary CTO is still the last barrier to complete percutaneous revascularization of patients with coronary artery disease. The presence of CTO, defined as an angiographic finding of complete occlusion of a coronary vessel of more than 3 months duration, is a common finding during routine coronary angiography, with a prevalence between 18% and 52% [6–11]. Despite high frequency of severe ischemia and angina resistant to medical therapy in most patients with CTO, the rate of attempted CTO PCI is low and averages 10%, with the Canadian multicenter registry showing wide variability in attempt rates ranging between 1 and 16% across different centers [11]. Based on the unpublished data from the Polish nationwide registry (ORPKI) on 535,857 patients treated with PCI between 2014 and 2018, only 12,572 (2.3%) patients underwent CTO PCI. This implies that patients selected for CTO PCI are often chosen according to the operator’s subjective opinion rather than the actual clinical need. Still, it is not uncommon for certain centers that patients with clear indication for revascularization are denied CTO PCI because of the absence of skills, tools or concerns in terms of the potential complications as well as non-adequate financial support of CTO procedures by the National Health Fund in Poland. At the other extreme, patients with simple CTO lesions, but no clear clinical indication for revascularization, are referred for CTO PCI. The lack of appropriate knowledge about the potential clinical benefits of CTO PCI, which is undeniably a more complex procedure with higher costs than the regular PCI of non-CTO lesions, has led to reluctance to refer these patients for percutaneous revascularization from the wider cardiology community [12].
Indications
Currently there is a modest level of evidence for the most optimal treatment of CTO. Significantly, patients with CTO lesions appropriate for intervention are those who are symptomatic (including both angina and/or dyspnea) despite optimal medical therapy (OMT). Hence, the European Society of Cardiology (ESC) guidelines assign CTO PCI a class IIa indication, whereby percutaneous revascularization of CTO should be considered in patients with angina resistant to medical therapy or with a large area of documented ischemia in the territory of the occluded vessel [13–19]. At the same time the authors of the ESC guidelines affirm that “broadly speaking, the treatment of CTO may be considered analogous to the treatment of non-CTO lesions” [13]. In addition, it is recommended that complex CTO procedures are only performed by adequately experienced interventionalists (class IIa, level of evidence C). However, there is ambiguity in the definitions of appropriate indications as well as sufficient “operator expertise”. In our opinion, PCI should be equally indicated in a patient with CTO (currently class IIa) as compared to a patient with a non-occlusive critical coronary stenosis (currently class I), provided that both lesions induce the same level of ischemia and symptoms.
Two recently published randomized controlled clinical trials and several observational studies reported symptoms improvement after successful CTO PCI [14, 20]. The EUROCTO trial randomized 396 patients in a 2 : 1 fashion to CTO PCI plus OMT or OMT alone. Significantly, all non-CTO lesions were treated before randomization to assign the intrinsic effect of revascularization to CTO. The success rate of CTO recanalization was 87%, and the trial showed superiority of PCI over OMT for angina frequency and quality of life at 12 months follow-up. In addition, physical limitation and functional angina class also improved in the CTO PCI arm [14]. The IMPACTOR-CTO trial included 94 patients with isolated CTO of the RCA and showed a significant decrease of ischemia burden as well as improvement of functional status and quality of life in the CTO PCI group versus the OMT group [20].
In contrast, two randomized controlled clinical trials showed neutral effects of CTO PCI. The EXPLORE trial randomized 304 patients after PCI for ST-segment elevation myocardial infarction (STEMI) to subsequent PCI or OMT for concomitant CTO. The primary endpoints were defined as left ventricular ejection fraction and left ventricular end-diastolic volume assessed by cardiac magnetic resonance at 4 months follow-up. Although the trial was negative, some methodological flaws (including slow recruitment and site volume with the mean of 2.4 patients/year/center, relatively low CTO PCI success rate of 73%, high crossover rate of 23%, and finally the lack of inducible myocardial ischemia test before PCI) might have influenced the results [21]. In lieu of these findings, the authors found that there were more patients with freedom from angina after CTO PCI at 1-year follow-up [22]. Another negative trial was the DECISION CTO trial, in which 834 patients were allocated to either CTO PCI or no CTO PCI. After 4.0 years of follow-up, there was no significant difference between the CTO-PCI and the no CTO-PCI strategies in the incidence of death, myocardial infarction, stroke, or any revascularization [23]. Nevertheless, the DECISION CTO trial was widely criticized due to its design (revascularization of non-CTO lesions was allowed in both groups and observed in more than 70% of patients, extremely low enrolment rate, 18% crossover rate, inclusion of all-cause death and stroke in the primary endpoint), and based on our opinion should not be taken into consideration when interpreting the potential benefits of CTO PCI [24].
In observational studies CTO PCI relieved regional ischemia and was associated with improved exercise capacity, increased anaerobic threshold, and fewer depressive symptoms [25–29]. Subsequently, it was proven that viable myocardium supplied by a CTO is a persistently ischemic zone [30, 31]. Both regional and global left ventricular function improved after successful CTO PCI in patients with viability or baseline dysfunction [32]. This result was, however, not confirmed in two randomized controlled trials [21, 33]. Nevertheless, these randomized studies were performed in patients with preserved left ventricular function, did not examine the presence of viable and dysfunctional myocardium, nor did they assess exercise induced changes in left ventricular function. Also, patients with a coronary CTO who received an implantable cardioverter defibrillator for primary or secondary prevention had a higher risk for ventricular arrhythmias as well as higher frequency of recurrent ventricular tachycardia after ablation than patients with non-occlusive coronary stenoses [34–36]. However, there are no randomized studies assessing whether CTO PCI may reduce the risk for subsequent arrhythmias. Importantly, in observational studies, patients presenting with acute MI (including both STEMI and non-STEMI) and a CTO in a non-culprit coronary artery had a higher risk of worse clinical outcomes as compared with patients without a CTO [37–39]. Also, a meta-analysis of 6 studies including 1253 patients showed that CTO PCI of the non-infarct-related artery in patients presenting with STEMI was associated with a significant reduction in major adverse cardiac events (MACE), cardiovascular mortality, and heart failure readmission [40].
In a meta-analysis of 25 observational studies comparing successful vs. failed CTO PCI, patients with a successful procedure had lower mortality [30]. Moreover, observational studies demonstrated a lower incidence of MACE after successful CTO PCI as compared with OMT alone irrespective of the quality of the collateral circulation [9, 41, 42]. Although CTO PCI might improve hard clinical outcomes, particularly among patients with a large ischemic burden (e.g. ischemia of > 10% of the myocardium) in whom complete revascularization is achieved [43–47], this hypothesis still requires confirmation in a well-designed, prospective, randomized clinical trial.
The belief that all CTO lesions are benign is likely unsubstantiated, and there are situations where intervention is highly indicated. Hence, a patient with CTO requires comprehensive assessment of symptoms (including arrhythmia), left ventricular function, ischemia, and/or viability prior to revascularization (Figure 1). In case of persisting angina and/or exertional dyspnea unrelated to other conditions (i.e. anemia or chronic obstructive pulmonary disease) and preserved left ventricular function, a high burden of ischemia is almost always present, and the referral for CTO PCI should be considered after meticulous assessment of the risk to benefit ratio.
Figure 1
Indications for CTO PCI according to symptoms, ischemia, and viability
CTO – chronic total occlusion, PCI – percutaneous coronary intervention.
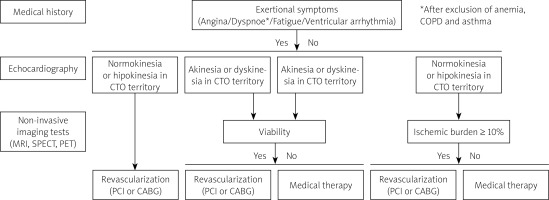
Of note, the decision to embark on CTO PCI should be discussed within the broader group of physicians including the physician in charge, non-invasive cardiologist and invasive cardiologist trained in CTO PCI. Equally important is a thorough discussion between a physician and a patient about the course and the length of the procedure, its potential complications as well as the expected clinical benefit.
Angiogram analysis
CTO PCI requires meticulous preparation. The first step includes careful and structured review of the angiogram, and also, if available, coronary computed tomography angiography (coronary CTA) [4]. It is generally discouraged to perform ad-hoc CTO PCI after diagnostic angiography so as to leave sufficient time for angiogram analysis, planning the procedure, and preparation of the availability of the catheterization laboratory as well as the whole team involved in the procedure. It is recommended to routinely perform dual catheter injection unless retrograde filling of CTO is absent via the donor artery [4]. Such an approach allows for better visualization and appreciation of CTO anatomy and prevents repetitive antegrade injections with increased risk of dissections during guidewire manipulation. According to the EuroCTO Club database, on average 75% of all CTO PCI are performed with dual catheter injection [5]. The review of angiogram should focus on 4 crucial CTO characteristics: 1) proximal cap morphology; 2) occlusion length, course and composition (visible calcium), 3) quality of distal vessel and distal cap morphology, 4) characteristics of the collateral circulation. Below we present the main rationale for the assessment of each of the above-mentioned angiographic characteristics:
Determination of the morphology of the proximal cap is crucial for selection of the most optimal approach – antegrade in case of a well-defined cap or retrograde in case of proximal cap ambiguity. Alternatively, additional imaging tools – such as intravascular ultrasound (IVUS) or coronary CTA (in selected cases with CT co-registration availability) or less often selective contrast injection via the microcatheter – can be employed for clarifying proximal cap ambiguity.
Lesion length can be accurately assessed by dual injection or coronary CTA. Both coronary calcification and vessel tortuosity can be better determined by coronary CTA than invasive angiography.
Distal vessel size as well as the presence of residual coronary artery disease and bifurcation at the distal cap should be routinely evaluated to gauge the chances of success in antegrade dissection and re-entry (ADR) technique.
Evaluation of collaterals is essential for deciding on a retrograde approach and should be obtained with high-quality angiography (optimally during breath hold and without panning) in different projections. Collateral size, tortuosity, bifurcations, entry and exit angles, as well as the distance from the collateral exit to the distal cap must be assessed. Lack of tortuosity and the optimal size increase the chances of successful guidewire crossing. Currently, the Werner classification is used to assess the quality of collaterals (CC0 – no continuous connection, CC1 – threadlike connection, CC2 – side branch-like connection) [48]. Selective contrast tip injection through a microcatheter using a 2 to 3 ml syringe can allow proper visualization of the collateral pathway. Septal collaterals are usually safe and relatively easy to cross using soft tip guidewires and can be dilated using small profile balloons in case of difficulty in microcatheter passage. The epicardial pathway should be reserved for very experienced PCI operators in adequately equipped centers prepared to treat epicardial collateral perforation with coils or other techniques (selective fat embolization, thrombin injection).
CTO scores
Currently, there is a large number of scores (including angiographic, computed tomographic and clinical parameters) which were developed to estimate the difficulty level of CTO. The historically first and most used scoring system is the relatively simple J-CTO score, which was originally validated to estimate the likelihood of successful and time-efficient (within 30 min) antegrade wire crossing. The J-CTO score is based on 5 variables: blunt proximal cap, bending > 45° within the CTO segment, occlusion length ≥ 20 mm, visible calcification, and previously failed attempt [48, 49]. Each present parameter is assigned one point and all points are summed to calculate the total J-CTO score and stratify lesion difficulty as: easy (J-CTO score of 0), intermediate (J-CTO score of 1), difficult (J-CTO score of 2), and very difficult (J-CTO score > 3 points). In addition, the J-CTO score has been validated to predict 1-year target lesion revascularization after successful CTO PCI [50]. Other angiographic scores to predict technical success of CTO PCI include: PROGRESS-CTO score [51], RECHARGE registry score [52], CL-score [53], ORA score [54], Ellis score [55], W-CTO score [56] and CASTLE score [57]. Also, it is endorsed to assess the risk of periprocedural complications by using the PROGRES CTO Complications Score [58].
Besides angiographic scores, coronary CTA-based scoring systems, including the CT-RECTOR score (Computed Tomography Registry of Chronic Total Occlusion Revascularization) [59] and the KKCT score (Korean Multicenter CTO CT registry score) [60], were developed. Noteworthy, both of these scores have superior diagnostic accuracy as compared with the angiographic J-CTO score for prediction of time-efficient guidewire crossing and final procedural success. Although coronary CTA is still rarely applied for planning and periprocedural guidance of CTO PCI, it surpasses invasive angiography for visualization of calcification, proximal cap ambiguity, tortuosity, and distal CTO segment [61]. Consequently, coronary CTA is particularly useful for guiding PCI in CTO lesions with poor visualization on invasive angiography such as long CTO, ostial occlusions, CTO in patients after coronary artery bypass grafting and previously failed CTO PCI. In addition, 3-dimensional CTA can be displayed and automatically aligned with the fluoroscopic C-arm in the catheterization laboratory so as to aid in: 1) selection of the most optimal angiographic projection with the least vessel foreshortening; 2) clarification of proximal cap ambiguity; 3) navigation of guidewire crossing along the occlusion site; and 4) facilitating the most optimal re-entry site in dissection and re-entry techniques. Currently, the wider application of coronary CTA for guiding CTO PCI is limited to a few luminary centers with availability of advanced angiography systems with in-built CT applications.
Access site
The choice of arterial access depends on the clinical situation and operator preference and can include various sites: bifemoral, biradial, radial and femoral, and least often two ipsilateral femoral sheaths. When choosing the femoral approach, it is advocated to use long (35 to 45 cm) and armed 7 Fr or 8 Fr sheaths. Although some operators prefer the radial approach (commonly using slender 7 Fr sheaths or less often regular 6 Fr sheaths) over the femoral approach, the benefit of reducing access site complications should be balanced against lesser supportive power of the smaller guiding catheters [62]. Hence, the radial approach for the donor artery and the femoral approach for the CTO vessel are frequently regarded as the most optimal solution for maintaining optimal support and reduction of arterial access complications.
Guiding catheters
CTO PCI requires optimal guiding catheter support (both passive and active) with coaxial alignment, and an adequately large lumen to host additional devices [5]. It is advised to use guiding catheters with side holes to prevent dissections unless ADR technique is contemplated as the most likely strategy. Most operators use 7 Fr guides or 8 Fr guides for the CTO vessel, and 7 Fr guides or 6 Fr guides for contralateral injection. For the left coronary artery extra backup guides (EBU) are preferred, although in some situations (such as CTO of the circumflex artery) the Amplatz guide can be advantageous. For the right coronary artery the Amplatz left guide is preferred as the most supportive guiding catheter, with other guides being used in specific anatomic situations (Judkins, Amplatz right, RBU, IMA, 3D RC, shepherd’s crook, hockey stick curves). The CTO PCI operators should be familiar with balloon anchoring and mother-in-child techniques.
Guidewires
During recent years substantial progress in guidewire technology has been achieved allowing for increased success rates of CTO recanalization despite growing lesion complexity [5]. Expertise in the composition and properties of the guidewires is mandatory for CTO PCI operators. Generally, guidewires can be divided into 3 categories according to the stiffness of its tip: soft (≤ 1 gr), intermediate (2–6 gr) and stiff (> 9 gr). Soft tapered polymeric composite core wires are intended for soft tissue tracking (passive wire control), intermediate tapered composite core guidewires are mostly applied for hard tissue tracking (active wire control), whereas stiff tapered wires are designed for calcified tissue penetration including highly resistant proximal cap crossing. In the current era, Fielder family wires (Asahi Intecc, Nagoya, Japan) including Fielder XT, XT-R and XT-A are mostly used for soft tissue tracking. In contrast, for active wiring (called the “deflection and rotation” technique) the Gaia family (Asahi Intecc, Nagoya, Japan) is preferred. The most commonly used stiff wires with high tipload/penetration force include Confianza Pro 12 (Asahi Intecc, Nagoya, Japan) and Hornet 14 (Boston Scientific, Marlborough, MA, USA). For the retrograde approach and collateral crossing, the preferred guidewires include Sion, Sion Black, Suoh 3 and Fielder XT-R (all Asahi Intecc, Nagoya, Japan). Subintimal tracking using the knuckle wire technique is usually performed with polymer jacketed guidewires such as Fielder XT or Fielder XT-A (both Asahi Intecc, Nagoya, Japan), Pilot 200 (Abbott Vascular, USA) or Fighter (Boston Scientific, Marlborough, MA, USA). Recently, the dedicated polymer jacketed and hydrophilic coating knuckle wire Gladius Mongo (Asahi Intecc, Nagoya, Japan) with increased lubrication and torque transmission has been introduced. An overview of currently available guidewires is presented in Table I.
Table I
An overview of CTO guidewires
Microcatheters
Microcatheters allow for safe delivery, enhanced navigation with adequate support and quick replacement of the guidewires and are essential for procedural success of CTO PCI [4, 5]. Specifically, the distance between the tip of the microcatheter and the CTO entry site can alter the penetration force, and thus the mechanical properties of the guidewire. Moreover, microcatheters allow not only for rapid exchange but also for multiple tip reshaping of the guidewires. Ultimately, following successful guidewire CTO crossing, microcatheters are advanced to the distal CTO segment beyond the occlusion site, enabling lesion dilatation and modification. In retrograde technique, microcatheters dilate collaterals channels and protect them from wire-induced trauma. In specific anatomic situations (parallel wire technique, side branch at proximal cap, bifurcation, wiring of difficult collaterals) dual lumen microcatheters can be successfully employed. Microcatheter selection depends on CTO angiographic characteristics, operator’s preference, and local availability. In contrast, over-the-wire balloons (still utilized by some operators due to financial constraints) are hampered by significant limitations (including difficulty in precise location of the tip, inefficient balloon advancement, and tendency to kink), and should not be routinely used in CTO PCI. In Table II the key characteristics of microcatheters used for CTO PCI are presented.
Table II
An overview of coronary microcatheters
CTO PCI techniques and hybrid algorithm
The main strategies in CTO PCI are classified as follows: 1) antegrade wire escalation, 2) ADR, 3) retrograde wire escalation, and 4) retrograde dissection and re-entry [4, 5].
Antegrade wire escalation is the most common initial CTO PCI strategy [2, 63–65]. It is based on different wires’ properties that are used according to CTO characteristics: (tapered stump or visible channels – polymer-jacketed low penetration force guidewires; blunt stump – intermediate and high penetration guidewires; highly resistant cap – high penetration guidewires). In each case escalation and de-escalation strategies are recommended depending on the particular situation. In case of subintimal tracking, parallel wire technique (optimally assisted by dual lumen microcatheter) can be implemented to avoid enlargement of the subintimal space [5]. Alternatively, ADR technique can be applied.
ADR technique can be either used as a first-choice CTO PCI strategy or in case of failed antegrade wiring or retrograde techniques [66]. The main concept of ADR is to enter the subintimal space, cross the occlusion site, and ultimately re-enter into the distal true lumen. The historically first ADR technique, called subintimal tracking and re-entry (STAR), is fully uncontrollable and can result in unnecessary stenting of long vessel segments with potentially higher risk for loss of the side branches and subsequent re-occlusion [67–69]. It was thus subsequently replaced with the “investment procedure” relying on subintimal plaque modification (using balloon predilatation) with delayed stenting for enhanced chances of antegrade wiring during repeat procedure [70, 71]. In contrast, the modern ADR relies on the application of dedicated re-entry systems such as the CrossBoss catheter and the Stingray balloon (both Boston Scientific, Marlborough, MA, USA) to minimize vascular injury, limit the length of the dissection and stenting segment, and, to some extent, preserve the side branches, leading to favorable clinical outcomes [72–75]. In addition, many other dissection techniques were developed to achieve successful re-entry into the true distal lumen (miniSTAR [76], limited antegrade subintimal tracking [77]) or penetrate the proximal cap (scratch and go, balloon assisted subintimal entry or Carlino [78]). Of particular interest, recently a novel and relatively simple ADR technique, called antegrade fenestration and re-entry, has been introduced, wherein multiple fenestrations are created at the level of the distal cap followed by advancement of a polymer-jacketed guidewire from the subintimal space into the distal true lumen [79, 80].
In the retrograde approach, the CTO is approached from the donor artery after advancing the guidewire and microcatheter through collaterals [81]. In some cases, it is feasible to cross the occlusion site into the true lumen (retrograde wire escalation technique), followed by guidewire externalization and subsequent conversion to the antegrade approach. In the majority of retrograde cases, however, the reverse controlled antegrade and retrograde tracking (reverse CART) technique has to be applied, wherein the balloon is inflated over the antegrade wire followed by retrograde wiring into the space created by the antegrade balloon, and subsequently the proximal true lumen of the CTO [3].
The integration of the above-mentioned techniques resulted in the concept of the “hybrid algorithm”, propagated by the North American operators [82]. Specifically, it is based on the premise that rapid switch of CTO PCI strategies in case of failure increases procedural efficiency. Noteworthy, such an approach is particularly salient in lesions with a high J-CTO score, wherein the chances of antegrade wire escalation fall to 30–40% [83]. The hybrid algorithm relies on the assessment of 4 angiographic features of CTO (proximal cap ambiguity, quality of the distal vessel, presence of interventional collaterals and occlusion length) that determine the initial PCI strategy and potential alternative techniques in case of failure. It has been confirmed that the application of the hybrid algorithm enhances procedural success rates (~90%) and safety [2]. After introduction of the hybrid algorithm, the Asia Pacific CTO Club algorithm was developed with some modifications (highlighted role of IVUS in proximal cap ambiguity, IVUS-guided wiring as a bailout strategy in the antegrade approach, use of parallel wire technique, use of CrossBoss catheter as a strategy of choice for in-stent CTO, and lack of CTO length as a determinant for choosing the initial antegrade strategy) [84]. Significantly, in 2019 the EuroCTO Club proposed a modified hybrid algorithm for CTO PCI, emphasizing the role of antegrade techniques to resolve proximal cap ambiguity (balloon assisted subintimal entry, scratch and go, IVUS-guided puncture) and giving equal relevance to ADR and parallel wire technique after failing antegrade wiring [5].
Cessation of CTO PCI
It is essential for minimizing complications and lowering the radiation dose to determine conditions for stopping CTO PCI in case of repeated failures. Hence, it is advocated to stop the procedure when there is no apparent progress of CTO PCI and: 1) procedural time is > 3 h, 2) contrast volume exceeds 4× the eGFR, or 3) the radiation dose is > 5 Gy [5]. In case of failure and planning a second attempt, CTO PCI should be re-attempted after 6 to 8 weeks. Although re-attempted CTO is associated with higher angiographic complexity as well as longer procedural and fluoroscopy time, in experienced hands the success and complication rates were reported to be similar as compared with the first attempt CTO PCI [85].
Drug-eluting stents and intravascular ultrasound
Drug-eluting stents are recommended after successful recanalization of CTO so as to reduce the rates of MACE, restenosis and re-occlusion as compared to bare metal stents [5]. While optimal stent sizing and expansion are paramount for improved long-term outcomes (including MACE and definite/probable stent thrombosis), the use of IVUS is particularly advocated in CTO PCI [86–89]. In addition, IVUS plays a pivotal role in the antegrade and retrograde approach for: 1) identification of the proximal cap, 2) antegrade reentry from the subintimal space, 3) retrograde guidewire crossing, 4) reverse CART technique [5]. In the EuroCTO Club database, the use of IVUS in CTO PCI increased from 5% in 2011 to almost 17% in 2019 (including 16% in antegrade and 23% in retrograde procedures) [90].
Pharmacology during and after procedure
For achieving optimal periprocedural anticoagulation an initial bolus (100 IU/kg) of intravenous unfractionated heparin (UFH) is routinely administered, and the activated clotting time (ACT) should be monitored every 30 min for the antegrade and every 20 min for the retrograde approach (optimally a dedicated nurse or a technician is assigned to monitor ACT). An additional bolus of UFH is administered to maintain ACT > 250–300 s during antegrade and > 350 s during retrograde procedure [5].
Dual antiplatelet therapy (DAPT) for at least 6 months following stenting is currently recommended in patients with stable ischemic heart disease (class I recommendation) [91]. Prolonged (up to 30 months) DAPT duration may be considered (class IIb recommendation) in patients after complex PCI [91]. Whereas the optimal duration of DAPT in patients after CTO PCI remains unknown, the tailored approach according to the clinical (diabetes, chronic kidney disease) and angiographic characteristics (e.g. number of stents, stent length, stent diameter) as well as the assessment of bleeding risk should be taken into account [13].
Complications and radiation issues
Despite the systematic decrease in CTO PCI-related complications, CTO PCI is associated with higher risk as compared with PCI of non-occluded coronaries [5]. Particularly, with the prevalence of 2.6–4.8% coronary perforations are the most common CTO PCI complications [92–94]. Significantly, most perforations occurring during CTO PCI are benign, and can be treated conservatively. The rate of tamponade is significantly lower and accounts for 0.4 to 1.3% of cases [2, 95]. Additional complications include: access site complications, donor vessel injury, arrhythmias, stroke, contrast-induced nephropathy, radiation dermatitis, emergency bypass grafting and death [96]. The average complication risk in most registries ranges from 0.5% to 7.0%, but high between-study variability exists [94, 97]. For example, whereas the rate of death rate was 0% in the UK Hybrid registry (1 156 patients), 0.2% and 0.9% death rates were reported in the EURO-CTO registry (17 626 patients) and OPEN-CTO registry (1 000 patients), respectively [94, 95, 98]. The potential measures to minimize the risk of complications include: 1) dual catheter injection for mitigating the risk of dissection and/or perforation by determination of the guidewire position, 2) placement of safety wire in the CTO donor vessel, 3) maintaining an appropriate ACT level for preventing donor vessel thrombosis, 4) availability of covered stents and coils in case of perforation, 5) proper hydration and limited contrast use (maximal amount of contrast defined as 4× of the pre-procedural eGFR) [4].
The reduction of the radiation dose encountered during CTO PCI should be the common goal of the whole CTO team. To this end, the ALARA protocol, with the aim of reducing radiation exposure to “As Low As Reasonably Achievable”, should be implemented in each catheterization laboratory. Particularly germane to this concept, the basic rules to limit radiation exposure include: 1) use of low-frame rate fluoroscopy and fluoroscopy-store function for documenting balloon and stent inflation instead of cine-angiography, 2) use of collimation, 3) reduction of the distance between the image receptor and the patient, 4) keeping the maximal possible distance from the X-ray lamp, and 5) proper use of protective shields [4, 7].
CTO program and operators’ requirements
Training in such complex procedures as CTO should start after the full understanding and exposure to PCI in general. Nevertheless, sufficient training in regular PCI does not automatically translate into an ability to become CTO operator.
High procedural volume is required to achieve and maintain CTO PCI skills [55, 99]. According to the Euro CTO Club, a large-volume catheterization laboratory with more than 1,000 PCI cases/year can provide continuous training to no more than 1–2 operators in order to exceed the minimal number of 50 CTO PCI cases per year/per operator. Since it is highly unlikely that the success rate will approach 80% in lower-volume centers, operators performing less than 30 CTO procedures annually should refer their patients to more experienced operators or involve proctors (particularly in CTO lesions with intermediate and high J-CTO scores) [5, 7]. It has been shown that while at least 100 CTO PCI/year must be performed by an operator to achieve a success rate > 90%, the minimal number to achieve a success rate > 80% approximates 50 CTO PCI/year [2, 5]. Retrograde techniques should be reserved for experienced operators (preferably those performing > 50 CTO per year). A minimum of 50 retrograde CTO PCI procedures (25 as a second operator and 25 as a first operator under supervision) might be advised before a cardiologist becomes an independent retrograde operator [7].
The minimal number of 50 CTO PCI per year to maintain competency translates into a model where only a limited number of operators and centers should perform CTO revascularization. The basic set of techniques for developing expertise in CTO PCI is represented by antegrade wire escalation and should be followed by implementation of retrograde techniques and ADR. Embracing the retrograde approach requires availability of specific devices (long microcatheters, dedicated wires, guide extensions, snares, etc.), learning several different techniques (from collateral channel engagement to wire externalization) and readiness to deal with complications (e.g. perforation, tamponade, donor vessel ischemia). Regarding collateral channel selection, it might be advisable to start tackling bypass grafts and septal channels first before embarking on retrograde CTO PCI via epicardial collaterals that represent the highest risk for life-threatening complications in case of rupture [5].
Hybrid algorithm according to the Association of Cardiovascular Interventions
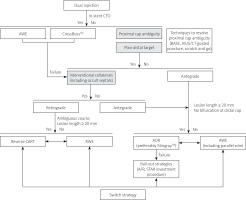
We have provided a modified hybrid algorithm (Figure 2) combining the main features of the original hybrid algorithm by Brilakis et al. and the EuroCTO Club hybrid algorithm [5, 82]. Similar to both of these algorithms we differentiate 3 main characteristics that determine whether the primary approach is antegrade or retrograde: 1) proximal cap ambiguity; 2) quality of the distal vessel; and 3) interventional collaterals. Uniquely, we: 1) specify the fundamental role of adjunctive coronary imaging (including IVUS and/or coronary CTA) as well as dedicated PCI techniques (balloon-assisted subintimal entry, scratch and go) to resolve proximal cap ambiguity and consequently avoid or improve the efficiency of retrograde procedures; 2) justify the discretionary use of the Crossboss catheter for crossing in-stent CTO; 3) acknowledge that both CTO length and ambiguous vessel course are equally important to dictate the choice of either a wire escalation strategy or a dissection and re-entry strategy; 4) put emphasis on parallel wiring (including IVUS guided re-entry) as a reasonable option after failing antegrade wiring; and 5) define ADR as an equal alternative to antegrade wiring in CTO lesions with clear or resolved proximal cap, occlusion length ≥ 20 mm and no major bifurcation at the distal cap. In addition, the use of ADR bail-out strategies (including antegrade fenestration and re-entry) has been underscored.
Figure 2
Modified hybrid algorithm for CTO crossing according to the Association of Cardiovascular Interventions of the Polish Cardiac Society
ADR – antegrade dissection and re-entry, AFR – antegrade fenestration and re-entry, AWE – antegrade wire escalation, BASE – balloon-assisted subintimal entry, CART – controlled antegrade retrograde tracking, CT – computed tomography, CTO – chronic total occlusion, IVUS – intravascular ultrasound, RWE – retrograde wire escalation, STAR – subintimal tracking and re-entry.
Reimbursement of CTO PCI
CTO PCI generally requires more procedural resources, and thus incurs higher procedural costs than regular PCI of non-CTO vessels. These costs are related to the use of multiple devices such as guiding catheters, guidewires, microcatheters (usually one or two depending on the use of retrograde approach), balloons, drug-eluting stents and closure devices, as well as specialized equipment such as dissection and re-entry catheters (CrossBoss catheter and/or Stingray balloon), guide extension catheters and intravascular ultrasound catheters, and to a lesser frequency atherectomy devices, coils, hemodynamic support devices or snares. This is particularly salient in percutaneous revascularization of CTO using the hybrid approach, wherein dual catheter injection and numerous modern CTO PCI techniques are applied ensuring high success rates exceeding 90%. Indeed, in a recent cost analysis among 964 patients from the OPEN-CTO registry, the procedural costs of CTO PCI using the hybrid algorithm ($12,280 ±5,972) were comparable to the costs of multivessel or left main PCI in the SYNTAX trial ($11,919 ±6,162) [100, 101]. Noteworthy, these costs did not account for other expenses such as nonprocedural hospital costs ($3,424 ±6,188) and physicians’ fees ($1,344 ±427) that further increase the total cost of CTO PCI in the United States ($17,048 ±9,904) [100]. Noteworthy, the total cost of CTO PCI calculated for 10 randomly selected patients from the National Institute of Cardiology in Warsaw in 2019 averaged PLN 15 505 ±6 169. Nevertheless, the cost of CTO PCI is still far lower than the cost of the alternate method of coronary revascularization, namely coronary artery bypass grafting (ranging between $44,824 to $448,038) in the United States [102].
Several countries have already provided higher reimbursement rates for CTO PCI than for regular PCI of non-CTO lesions to account for the additional costs incurred during percutaneous recanalization of CTO. For example, in Germany the reimbursement rate for CTO PCI using either an antegrade or retrograde approach amounts to €4,500 and exceeds the maximal reimbursement rate for non-CTO PCI (€3,500). Furthermore, in Japan and Singapore separate reimbursement for the devices used during the CTO PCI procedure (microcatheters, guidewires, balloons, stents, etc.) is secured. This is in contrast to the situation in Poland, where all CTO PCI are substantially underestimated and calculated as regular PCI. This, in turn, translates into excess expenditure for the still low number of healthcare providers offering CTO PCI, and further restrains guideline-recommended interventions among patients with CTO in Poland. The potential solution for this issue at a national level might rely on the institution of CTO PCI reference centers specialized in the most difficult CTO interventions (e.g. in lesions with J-CTO score ≥ 2).
Expert recommendations of the Association of Cardiovascular Interventions
Symptom improvement (including effort angina, dyspnea, and fatigue) is the primary indication for CTO PCI (based on randomized clinical trials). The secondary indications for CTO PCI include improvement of clinical prognosis, increase in left ventricular systolic function and reduction of malignant arrhythmias (based on observational data and expert opinions only).
Referral for CTO PCI should be mainly based on clinical grounds (symptoms) as well as ischemia and/or viability assessment. The clinical benefit of CTO PCI should be balanced against the procedural risk related to the specific clinical and angiographic characteristics.
Dual coronary angiography and meticulous analysis of the angiogram (and if available coronary CTA) are paramount for planning and safe recanalization of CTO.
The use of microcatheters is mandatory to enhance guidewire manipulation and ensure procedural safety.
There are 4 main strategies for CTO PCI: antegrade wire escalation, antegrade dissection and re-entry, retrograde wire escalation, and retrograde dissection and re-entry. The full-skilled CTO operator must not only be familiar with all of these techniques, but also quickly change from one technique to another in case of failure.
A hybrid algorithm has to be applied when planning and performing CTO recanalization.
Radiation and contrast safety issues must be meticulously taken into account when planning and performing CTO recanalization.
Dedicated CTO training and adequate procedural volume are essential to ensure high success and low complications rates. Operators embarking on the CTO PCI training program should start with easy and intermediate CTO lesions (J-CTO score of 0 and 1) and involve proctors in more difficult cases (J-CTO score ≥ 2). Centers with a low number of CTO PCI (< 30 yearly) should refer difficult CTO cases to specialized CTO centers. The CTO operator must perform at least 50 procedures annually to maintain skills and reduce the risk of complications.
The use of IVUS during CTO PCI is recommended for reduction of the risk of restenosis and re-occlusion and improvement of long-term clinical outcomes.
There is unmet need for an adequate number and high quality of CTO procedures in Poland, which are vastly underused due to significant financial constraints.
Summary
Coronary CTO are increasingly encountered during invasive and non-invasive coronary angiography. Noteworthy, most patients with CTO have inducible ischemia and are symptomatic despite optimal medical therapy, rendering them ideal candidates for coronary revascularization. Historically, based on its angiographic complexity, the presence of CTO was the key driver for coronary artery bypass grafting or medical therapy only. During recent years, however, a clear shift towards percutaneous treatment of CTO has been witnessed, particularly as a result of the unprecedented progress in CTO PCI techniques and equipment as well as specialized training programs of CTO operators. In this regard, high success rates (exceeding 90% in some luminary centers) along with improved safety outcomes of CTO PCI can be achieved. Significantly, based on randomized clinical trials CTO PCI has been shown to relieve angina and enhance exercise tolerance, translating into improved quality of life. Nevertheless, and contrary to the Western European countries, CTO PCI is significantly underused and accounts for less than 3% of all PCI in Poland, leaving many patients with CTO untreated and symptomatic. This mainly results from the barriers in obtaining the adequate skills, tools and training programs among Polish CTO operators as well as non-adequate financial support of CTO procedures in Poland.
The current expert consensus document summarizes the rationale, clinical outcomes as well as technical and safety issues of CTO PCI. Significantly, we have presented a modified hybrid algorithm (the so-called Polish hybrid algorithm) providing some unique refinements to the contemporary CTO PCI strategies. Finally, a list of succinct and practical recommendations on CTO PCI has been provided. Continuous efforts of scientific societies and national associations (including active engagement with the payer) are urgently needed to expand CTO PCI knowledge and training programs and close the gap between Poland and Western European countries.








