Introduction
Breast cancer (BC) is the most common type of non-epidermal cancer and the second leading cause of cancer-related deaths in women worldwide. Although BC treatment has been improved, the 5-year survival rate is still less than 50%, with an increased post-operative relapse rate due to the presence of undetectable cancer cells – these cells in the peripheral blood may play a role in the recurrence of the disease either locally or in distant places [1–3].
Cancer treatment has become an important field for molecular studies along with vital mechanisms of cancer progression. It is necessary for researchers to recognize novel biomarkers or drugs that help to overcome cancer [4].
In the early course of BC, the spread of malignant cells from the primary tumour through the lymphatics or the blood occurs, which leads to local recurrence or distant metastasis. Hence, the detection of circulating tumour cells (CTCs) in BC patients (non-metastatic or metastatic) is related to unfavourable prognosis [5].
Circulating of tumour cells (CTCs) in the blood are considered a significant prognostic indicator in both primary and metastatic BC patients. It can be assessed at any stage of the disease; thus, checking their level may be crucial for follow-up evaluation and monitoring the effect of treatment [6]. The association between CTCs and unfavourable prognosis may provide a unique tool to determine the prognostic and predictive significance and to monitor BC recurrence.
Cytokeratin 19 (CK19) is a specific epithelial cytoskeleton marker that is used to detect tumour cells in the peripheral blood, bone marrow, and lymph nodes of cancer patients; it is highly expressed in epithelial tumours such as BC [7].
Immune phenotyping by flow cytometry, in the peripheral blood samples of the early and late stages of the BC can be used as a reliable method for the detection of cytokeratin 19 (CK19) [8].
Hence CK19 is considered as a strong and reliable epithelial tumour marker, and it is used to diagnose and evaluate the prognosis of various cancers of epithelial origin [9].
Cancer stem cells (CSC) or tumour-initiating cells possess properties of self-renewal, which causes an increase in treatment resistance and promotion of metastasis, in turn resulting in an increased relapse rate [10].
There are several markers that have recently been reco-gnized as stem cell biomarkers. Octamer-binding transcription factor 4 (OCT4), also known as POU5f1, is a transcription factor of the POU family that plays a highly significant role in self-renewal and maintenance of pluripotency in embryonic stem cells, and it has been used as a promising CSC marker [11]. Moreover, it has been shown that the high reactivity of OCT4 is associated with cancer cells’ aggressive behaviour; therefore, it can be used as a potential biomarker for predicting poor prognosis in different malignancies [12]. However, the prognostic value of CK19 or OCT4 overexpression in BC remains unknown and lacking in clinical data support, especially in non-metastatic BC.
The aim of this work is to evaluate the prognostic significance of CK19 detection in peripheral blood, and OCT4 expression in tissues of BC patients, and their relation to the patients’ outcome.
Material and methods
Study population
This prospective cohort study was conducted on 70 patients: 61 patients with BC (44 of them were non-metastatic and 17 patients with metastasis) and 9 patients diagnosed with tumour, who were considered as a negative control group, during the period between October 2016 and October 2019. Our BC patients were admitted, operated, and treated in the Department of General Surgery, Department of Clinical Oncology and Nuclear Medicine, and Department of Medical Oncology of Zagazig University. Peripheral blood samples were obtained in the Department of Clinical Pathology of Zagazig University: from non-metastatic BC patients (before the beginning of treatment), from metastatic BC patients (before the first cycle of the first-line chemotherapy), and from 9 patients with benign tumours (negative control).
Tissue samples, including diagnostic true cut biopsy or after breast conserving surgery (BCS) or modified radical mastectomy (MRM), were sent to the Department of Pathology of Zagazig University, where samples were processed, diagnosed, graded, and staged. The seventh edition of the American Joint Committee on Cancer staging system (AJCC-7) classification 2010 and the World Health Organization (WHO) classification were used for pathologic staging and grading, respectively [13].
Patients with good Eastern Cooperative Oncology Group (ECOG) performance status (0–2) were included in our study. Those who had inadequate organ or bone marrow functions were excluded.
Clinical and pathological criteria such as tumour size, age, grade, sex, and stage were recorded. The hormonal receptors were evaluated: oestrogen receptor (OR) and progesterone receptor (PR), and Her2/neu expressions and Ki67 labelling index for all breast cancer cases were included in our study. Both ER and PR were considered positive if more than 1% of tumour cells had positive nuclear staining [14]. HER2/neu 3+ or equivocal 2+ with amplification on in situ hybridization (ISH) was considered positive. High Ki67 labelling index was identified as > 14% while low index was < 14% [15].
The treatment was given to non-metastatic patients (chemotherapy, radiotherapy, and hormonal treatment with or without trastuzumab) according to their indication and staging; nevertheless, the palliative systemic treatment and radiation therapies were given to metastatic cases according to their indication.
All participating patients in our study gave written informed consent, and the study was approved by our institution’s Ethical and Scientific Committee.
Blood samples
Peripheral blood samples (3 ml) were collected from each patient under complete aseptic conditions in ethylene-diamine tetraacetic acid di-potassium (EDTA K2) tubes (K-vacutest) at a final concentration of 1.5 mg/ml for complete blood count and flow-cytometric analysis. Samples were taken as follows: from 44 non-metastatic BC patients before the beginning of treatment, from 17 previously untreated metastatic BC patients before the beginning of first-line chemotherapy, and from 9 patients with benign tumours as control samples.
As regards flow cytometry (FCM), CK19 from the blood samples of BC patients and control were enumerated. They were detected by fluorescein isothiocyanate conjugated CK19 FITC and peridinin-chlorophyll-protein-conjugated CD45 PE; all reagents were purchased from BD Biosciences (San Jose, USA). For EDTA blood samples, erythrocytes were lysed by adding lysing solution followed by adding 10 µl of CD45 to the suspension of the cells. After the samples were incubated in the dark for 20 minutes, they were washed with phosphate-buffered saline (PBS). This step was followed by the addition of fixative solution for 15 minutes to fix the cells. Cells were washed with phosphate buffer saline PBS, and then 10 µl of CK19 was added with its permeabilizing solution followed by incubation at room temperature for 15 minutes; subsequently, it was washed with phosphate buffer saline (PBS) to be ready for analysis.
Data were analysed by Cell Quest software using an FACS Calibur instrument, Becton Dikinson Immune cyto-metry Systems (San Jose, CA, USA). At least 50,000 cells were acquired to identify CD45 (PE) and CK19 (FITC) co- expressions. An isotype-matched negative control antihuman IgG was used [16].
Immunohistochemical staining
This study included sections from formalin-fixed, paraffin-embedded samples retrieved from BC patients and from patients with benign breast tumours. Immunohistochemistry was performed as previously explained [17]. Sections with primary mouse monoclonal anti-OCT4 antibody were incubated (1 : 200 dilutions, anti-OCT4 antibody [ab18976] Abcam, USA).
Evaluation of OCT4 immuno-histochemical expression in stained tissues
Nuclear expression of OCT4 was considered positive. Staining intensity was assessed and divided into the following scores: negative stain 0, light yellow stain = 1, dark yellow stain = 2, and dark brown stain = 3. Staining extent was assessed and divided into the following 5 grades: stained cells = 0 if stain was less than 10%, between 10–25% = 1, 25–50% = 2, 50–75 = 3, and > 75% = 4. Finally, the values were multiplied to reach scores from 0–12. Six was established as a cut-off point above which OCT4 expression was defined as high, and below which OCT4 expression was defined as low [11].
Statistical analysis
The collected data were entered into a computer and statistically analysed using SPSS (Statistical Package for Social Science) version 24. Qualitative data are represented as frequencies and relative percentages. Quantitative data are expressed as mean ± SD (standard deviation). Chi-square test (χ2) and Fisher’s exact were used to calculate differences between qualitative variables, as indicated. Independent t-test was used to calculate difference between quantitative variables among 2 groups. All statistical comparisons were 2 tailed with p ≤ 0.05 indicating a significant difference, p < 0.001 highly significant, and p > 0.05 indicating a non-significant difference.
Survival analysis: The Kaplan and Meier method was used to estimate overall survival and progression-free survival (PFS) rates, while the log rank test compared survival curves. Overall survival (OS) was calculated as the time from diagnosis until death or the most recent follow-up contact (censored); meanwhile, PFS was calculated from start of the treatment to either progression or the most recent follow-up contact when the patient was known to be relapse-free. Stratification of OS and PFS was done according to markers.
Results
Sixty-one BC patients (44 patients with non-metastatic BC, 17 patients with metastasis, and 9 with benign tumours) were included in this study. Their mean age was 48.0 ± 9.8 years. At time of the study, 40 patients (65.6%) were diagnosed as invasive ductal carcinoma (IDC, NOS), 14 patients (23.0%) had invasive lobular carcinoma (ILC), and 7 (11.5%) had other histopathological subtypes. Lymph node (LN) metastases were present in 51 patients (83.6%). CK19 was detected in 62.2% while OCT4 was positive in 60.6% of patients. The clinicopathological characteristics of the studied subjects are shown in Table 1.
Table 1
The clinicopathological features of the entire group of patients (N = 61)
Relationship between CK19 and clinicopathological features (Tables 2, 3 and Fig. 1)
Fig. 1
Flow cytometric histogram analysis in breast cancer patients and control, all samples were labeled with antibodies against CD45 PE and CK19FITC. CK19 expression in control (A), CK19 expression in stage II (B), CK19 expression in stage III (C), CK19 expression in stage IV (D)
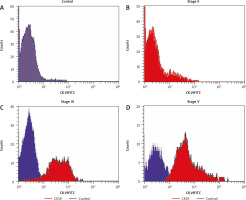
Table 2
CK19 and OCT4 expression in breast cancer and benign tumours
| CK19 | p-value | OCT4 | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative, n (%) | Positive, n (%) | Negative, n (%) | Positive, n (%) | |||||||
| Breast cancer | 23 | 37.7 | 38 | 62.2 | < 0.001 | 24 | 39.30 | 37 | 60.60 | < 0.001 |
| Benign tumour | 9 | 100.00 | 0 | 00.00 | 9 | 100.0 | 0 | 00.00 | ||
Table 3
Association of clinical features with expression of markers in the studied population (N = 61)
Although the expression level of CK19 was significantly high in BC patients, it was not detected in patients with benign tumours. It was detected in 38 patients with BC (62.2%), 13 patients with metastasis (34.2%), and 25 (65.8%) with no metastasis, and they were positively associated with high grade (p = 0.002), HER2 positivity (p = 0.009), metastasis (p = 0.026), molecular subtypes, LN positivity, and advanced stage (p = 0.001). No statistically significant association was found between CK19 expression and age, menopausal status, body mass index (BMI), family history, diabetes mellitus (DM), pathological types, capsular invasion, ER, PR, KI67, or T.
Relationship between OCT4 and clinicopathological features (Tables 2, 3 and Fig. 2)
Fig. 2
Immunohistochemical expression of Oct4 in carcinoma of the breast. High expression in the nucleus of high grade infiltrating lobular carcinoma of the breast stage IV ×400 (A), high expression in the nucleus of high grade infiltrating duct carcinoma of the breast stage IV ×400 (B), high expression in the nucleus of high grade infiltrating duct carcinoma of the breast stage III ×400 (C), low expression in the nucleus of low grade infiltrating duct carcinoma of the breast stage I ×400 (D), low expression in the nucleus of low grade infiltrating lobular carcinoma of the breast stage II ×400 (E)
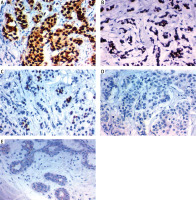
The expression level of OCT4 was significantly higher in samples of BC patients than in control patients with benign tumours (p < 0.001). None of the benign tumour patients had positive OCT4 expression. It was positive in 37 patients (60.6%), 24 non-metastatic patients (64.8%), and 13 (35.1%) metastatic patients. Interestingly, it was positively associated with high BMI (p < 0.023), aggressive molecular subtype (p < 0.019), positive ER expression (p = 0.025), presence of LN metastases (p < 0.017), and distant metastasis (p < 0.018).
No statistically significant association was found between OCT4 expression and DM, age of the patient, family history, grade, menopausal status, presence of capsular invasion, tumour stage, T, positive PR expression, positive Her2-neu expression, high Ki67 labelling index, or histopathological subtype of the tumour.
Association between expression of CK19 and OCT4 (Table 4)
Table 4
Co-expression of markers in the studied population
| CK19 | Total, N = 61 | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative, n = 23 | Positive, n = 38 | |||||||
| n | % | n | % | n | % | |||
| OCT4 | Negative | 11 | 47.8 | 13 | 34.2 | 24 | 39.3 | 0.291 |
| Positive | 12 | 52.2 | 25 | 65.8 | 37 | 60.7 | ||
A non-significant association was found between the expression of CK19 and OCT4 (p = 0.291).
Outcome of patients in relation to marker expression (Tables 5, 6 and Figs. 3–5)
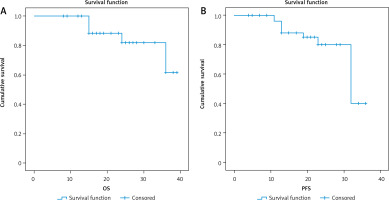
Fig. 4
3-year overall survival (OS) and 3-year progression-free survival (PFS) as regard CK19 expression
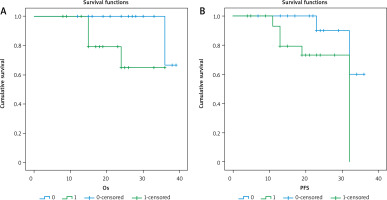
Fig. 5
3-year overall survival (OS) and 3-year progression-free survival (PFS) as regard OCT4 expression
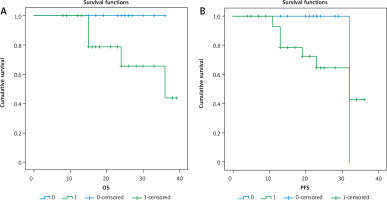
Table 5
Clinical outcomes of patients in relation to expression markers
Table 6
Mean survival time and survival rates in relation to each marker
After a median follow-up period of 21 months with range of (8–39) months, there was no significant relationship between positive expression of CK19 and local recurrence (p = 0.441), progression (p = 0.727), or death (p = 0.075). There was a significant inverse relationship between positive expression of CK19 and both 3-year overall survival (OS) and 3-year progression-free survival (PFS) (p = 0.011 and 0.026), respectively.
There was a significant relationship between positive expression of OCT4 and the presence of progression or death (p = 0.014 and 0.009, respectively), with a significant inverse relationship between OCT4 positive expression and 3-year OS (p = O.004) or 3-year PFS (p = 0.027).
Discussion
Detection of CTCs in patients with non-metastatic or metastatic BC is associated with tumour progression, lack of treatment efficacy, and unfavourable prognosis [18]. BC cells express CK 19, which is commonly used as a molecular marker for CTCs. Therefore, CK19 is a marker of different epithelial cells that are subjected to premalignant, malignant, or metastatic transformation [19].
OCT4 is associated with aggressive behaviour of cancer cells, and it can be considered a potential biomarker for predicting poor prognosis in different malignancies [12]. This study was designed for the evaluation of CK19 and OCT4 as a survival marker for non-metastasized or metastasized BC patients.
In our study, CK19 was detected in 38 (62.2%) BC patients: 25 (56.8%) non-metastatic and 13 (76.4%) metastatic patients, but no CK19 expression was found in the control group. These findings are in accordance with Wang et al. [16], who showed that CK19 is significantly higher in BC patients.
Also, our findings showed that CK19 expression was significantly associated with advanced stage III (55.3%) and IV (34.2%) and molecular subtypes. This agrees with the study of Keyvani et al. [6]. These data also agree with Wang et al. [16] and Keyvani et al. [6], who showed increased CK19 expression with progression of stage of the disease. However, Stathopoulou et al. [20] found that CK19 is highly expressed in the peripheral blood of patients with early BC.
Our study showed significant association between CK19 and LN metastasis. This concurred with the study by Yu et al. [21], who found a significant relationship between CK19 expression in peripheral blood of breast cancer patients and the number of positive LNs. On the other hand, Park et al. [22] failed to detect a significant association between CK19 expression and the number of LN metastases. This may be attributed to the difference in the method of detection.
Furthermore, our study showed strong association between the expression of CK19 and distant metastasis. These results are in accordance with Alvarenga et al. [23]
In addition, OCT4 expression was associated with BC, which was detected in 37 BC patients (60.6%); it was posi-tive in 64.8% of non-metastatic and 35.1% of metastatic patients. Its expression was higher in malignant than in non-malignant patients. OCT4 was positively correlated with BMI, the presence of LN metastases, distant metastasis, aggressive molecular subtype, and positive ER expression.
Gawk et al. [24] demonstrated that OCT4 was highly expressed in BC with aggressive molecular subtypes, which is in accordance with our results.
Joshi et al. [12] reported that OCT4 expression was not associated with tumour size or grade, which matched with our results. On the contrary, Liu et al. [25] showed signi-ficant association between OCT4 expression and tumour size and grade, which might be attributed to different detection methods, sample size, and ethnicity.
OCT4 is thought to play an important role in the epithelial-mesenchymal transition (EMT) process. In hepatocellular carcinoma, knockout of OCT4 reduced the proliferation rate and reversed EMT [26]. On the other hand, Hu et al. [27] demonstrated that silencing OCT4 promoted the invasiveness and spread of BC cell line MCF-7 by inducing EMT. This may imply a complex regulatory loop between OCT4 and EMT signals in breast cancer [28].
After a median follow-up period of 21 months with a range of 8–39 months, we found a significant inverse association between CK19 expression and the 3-year OS and 3-year PFS. These findings are in accordance with Aaltonen et al. [29], who stated that blood CK19 expression could predict the disease progression and death of BC patients. Similarly, Park et al. [22] found that CK19 detection was associated with poor overall survival in univariate analysis.
Xenidis et al. [30] revealed that CK19 might be a useful marker for taking a therapeutic decision in BC patients and should be used in the context of clinical trials.
Saha et al. [31] attributed the high expression of CK19 in BC patients and its association with its invasiveness to the knockdown of CK19, which led to increased proliferation, migration, invasion, and drug resistance.
We also obtained a significant association between positive expression of OCT4 and both disease progression and death, with a significant inverse relation between its positive expression and 3-year OS or 3-year PFS. These results are supported by Gwak et al. [24], who found that OCT4 might be an independent negative prognostic factor in BC patients, especially in the hormone receptor-positive subgroup and in those who received tamoxifen. Furthermore, Joshi et al. [12] stated that OCT4 expression was associated with reduced OS; they also found, using multivariate analysis, that OCT4 was a significant independent prognostic factor for predicting OS in BC.
Our results were supported also by the study done by Yang et al. [11], who stated that OCT4-positive expression was associated with worse OS in HER2+ BC patients; this might be due to the impact of OCT4 on cancer cell functions such as proliferation, migration, invasion, and metastasis via mediating multiple pathways to increase disease severity in addition to the chemo resistance. Similar results were obtained by Zhang et al. [32], who detected a significant association between OCT4 in BC and survival.
Although we found a non-significant association between CK19 and OCT4 expression, each of them was significantly associated with poor survival in BC patients; hence, they can be considered useful prognostic and predictor markers in BC patients.
The limitations of this study include the small sample size and short follow-up period. Hence, we recommend further studies with larger patient numbers and longer follow-up periods. Also, the use of different adjuvant and first-line chemotherapy protocols might have affected the tumour outcomes.