Introduction
The NOD2 protein plays an important role in the function of the immune system. It is active in certain types of immune system cells, including monocytes, macrophages, and dendritic cells, which help to protect the body against foreign invaders, such as viruses and bacteria [1]. NOD2 also regulates the activity of genes that control immune responses and inflammatory reactions, and plays a role in a process called autophagy [3]. The gene that encodes this protein is located on 16q12.1 [2].
The NOD2 3020insC allele is reported in ~7.3% of the Polish population [4]. In the literature, the presence of NOD2 mutation is associated with increased risk of breast cancer (BC) before the age of 50 years (~1%) [5]. This mutation occurs in ~8% of all BC and increases the risk of DCIS in those aged < 50 years by five times. The presence of the NOD2 3020insC allele also increases the lifetime risk of colorectal cancer at the age of over 60 years (by over 2-fold), lung cancer (~2-fold), and ovarian cancer (~1.5-fold) [6, 9]. The 3020insC mutation of the NOD2/CARD15 gene may also be a genetic predisposing factor for aggregations of breast and lung cancer [11].
In the literature, there are only a few data describing the association between the presence of NOD2 mutation and clinicopathological factors. The associated factors confirmed in the aforementioned studies were younger age at diagnosis, family history of cancer (especially breast and gastrointestinal cancer) [8], and early-stage disease [6]. In our previous study, the presence of NOD2 (3020insC) in women with BC was characterised by positive predictive factors such as: lymph nodes without metastasis (N0), lower histological grade (G<3), and negative HER2 receptor status (HER2–) [7].
The purpose of the present study was to characterise BC NOD2-mutation carriers at age ≥ 50 years, according to clinicopathological factors or family history. Patients aged ≥ 50 years were compared with the control group and with NOD2-mutation carriers at age < 50 years.
Material and methods
The analysed group were divided into two subgroups according to patient age: 1) < 50 years; and 2) ≥ 50 years. The clinicopathological factors were analysed, as well as the presence of cancer in family history in younger (age, < 50 years) (45.3%, n = 68/150) and in older (age, ≥ 50 years) (54.7%, n = 82/150) patients with BC with confirmed NOD2 (c.3020insC) mutation (GenBank NM _022162.1). Control groups were selected from patients with BC who tested negative for the mutations (37.5% < 50 years, n = 141; 62.5% ≥ 50 years; n = 235/376). The presence of the most common mutations in BRCA1 (c.68_69delAG, c.181T>G, c.4034delA, c.5266dupC, c.3700_3704del5) (GenBank NM_007294.3), BRCA2 (c.5946delT and c.9403delC) (GenBank NM_000059.3), and CHEK2*1100delC or I157T (GenBank NM_007194.3) genes were excluded. Mutation analysis was carried out by a multiplex allele-specific PCR assay. Genetic diagnostics were conducted between the years 2012 and 2018. All patients gave written, informed consent for genetic examination.
All patients were females who were diagnosed, treated, and followed-up at the National Research Institute of Oncology in Gliwice. Patients underwent clinical follow-up examinations every three months in the first two years, every six months afterwards until the fifth year after diagnosis, and every year subsequently. The inclusion criteria were: BC confirmed by microscopic examination; performance status ZUBROD 0–1; age above 18 years; the normal levels of renal and liver function and normal values of bone marrow. The data of age at onset, menopausal status, surgical procedure, disease stage according to TNM classification, histology, oestrogen (ER) and progesterone receptor (PR) status, HER2 status, and contralateral BC were gathered from hospital records and pathology reports. The analysis of patient medical records was performed according to national law regulation.
The median age at diagnosis of all patients was 52.4 years (range, 25.5–80.7 years). Patient clinicopathological characteristics are presented in Table 1. A total of 209 patients were at the age < 50 years and 317 at the age ≥ 50 years. All patients had good performance status (ZUBROD 0–1). The complete characteristics of patients with regards to demographic and clinicopathological features are presented in Tables 2 and 3.
Table 1
Clinicopathological characteristics of all study group breast cancer patients (n = 526)
Table 2
Clinical characteristics of breast cancer patients according to age
Table 3
Pathological characteristics of the tumours in breast cancer patients according to age
Statistical analysis was carried out using Statistica13 software. The qualitative features were presented as the percentage of their occurrence and evaluated with Fisher’s test and the c2 test with Yates’ correction. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated as measures of association between the analysed factors and the presence of NOD2 mutation. The differences were considered significant if the p-value was < 0.05.
Results
The median age at BC diagnosis for the carriers of the NOD2 mutation was 51.4 years (range, 25.5–80.7 years) and 53.2 years (range, 26.4–78.3 years) for the control group. A total of 209 patients were aged under 50 years: 68 (45.3%) were NOD2-mutation carriers and 141 (37.2%) were in the control group.
Group of patients aged ≥ 50 years
In the group of patients ≥ 50 years old, a family history of cancer, including BC, was observed more often in NOD2-mutation carriers compared with the control group of patients (65% vs. 52%; OR = 1.66; p = 0.072, with family history of BC vs. no family history of BC; 32% vs. 15%; OR = 2.65; p = 0.002). There were no differences between the control group and NOD2-mutation carriers at age ≥ 50 years, in those with a family history of other cancer types.
In the group aged ≥ 50 years, lower tumour size (T1–T2) was observed more often in NOD2-mutation carriers compared with the control group (94% vs. 82%; OR = 3.35; p = 0.011). Similarly, in patients aged ≥ 50 years, lymph nodes without metastases were reported insignificantly more frequently in NOD2-mutation carriers (67% vs. 58%, OR = 1.48, p = 0.182). Conversely, histological grade G3 tumours (17% vs. 30%, OR = 0.49, p = 0.036) and HER overexpression (10% vs. 49%, OR = 0.11, p = 0.0001) were present significantly less often in NOD2-mutation carriers. There was also a significant difference between NOD2-mutation carriers and the control group according to ER– (16% vs. 32%; OR = 0.39; p = 0.007) and PR– (23% vs. 43%; OR = 0.41; p = 0.003) negative steroid receptor status in the group of ≥ 50 years. In the study group, there was no notable difference between NOD2-mutation carriers and the control group, according to the BC histological type (Table 3).
Group of patients aged < 50 years
In the group of patients < 50 years old, there was no difference observed between NOD2-mutation carriers and the control group, according to those with a family history of cancer (60% vs. 59%; OR = 1.06; p = 0.963), particularly those with a family history of BC (25% vs. 19%; OR = 1.41; p = 0.429). Conversely, history of colorectal cancer was observed significantly more often in younger NOD2-mutation carriers compared with the control group (15% vs. 6%; OR = 2.87; p = 0.037). A similar tendency was reported in those with a family history of pancreatic cancer (5.9% vs. 1.4%; OR = 4.34; p = 0.089). There were no differences between younger patients and the control group, based on a family history of stomach cancer (8.8% vs. 8.5%; OR = 1.04; p = 0.851). Similar results were reported in those with a family history of lung cancer (8.8% vs. 9.2%; OR = 0.95; p = 0.870) or gynaecological cancer (10.3% vs. 8.5%; OR = 1.23; p = 0.870) (Table 2).
There was no difference between younger NOD2-mutation carriers and the control group, according to tumour size (T1–T2) (84% vs. 82%; OR = 1.12; p = 0.934). Lymph nodes without metastases (63% vs. 47%; OR = 1.95; p = 0.038) were significantly more frequently observed in NOD2-mutation carriers compared with the control group at this age. In the group of individuals of < 50 years old, histological grade G3 was non-significantly more often observed in NOD2-mutation carriers (p = 0.658). Conversely, HER2 overexpression was reported significantly less often in NOD2-mutation carriers (22% vs. 54%; OR = 0.24; p = 0.0001) at this age. There was a significant difference between NOD2-mutation carriers and the control group, according to ER– (24% vs. 38%; OR = 0.50; p = 0.049) negative steroid receptor status, while there was no differences for PR– (31% vs. 40%; OR = 0.68; p = 0.277) negative steroid receptor status. Conversely, there was no difference between NOD2-mutation carriers and the control group, according to BC histological type – ductal invasive carcinoma (79% vs. 78%) in the group of individuals < 50 years (Table 3).
Breast cancer molecular subtypes
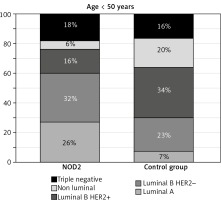
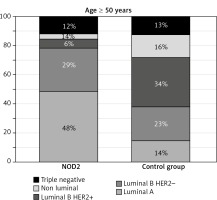
The presence of BC molecular subtypes in NOD2-mutation carriers and in the control group of patients differ from each other significantly (p = 0.0001). This is due to the differences in the presence of HER2 overexpression, positive oestrogen (ER+), and progesterone (PR+) steroid receptor status in both groups (NOD2-mutation carriers and the control group). The most commonly observed BC subtypes in the present study among NOD2-mutation carriers were luminal A and luminal B HER2(–). These subtypes were observed in 60% of younger NOD2-mutation carriers (< 50 years) and in 78% of NOD2-mutation carriers over 50 years of age (p = 0.018). In the group < 50 years of age, luminal A (26% vs. 7%) and luminal B HER2(–) (34% vs. 23%) BC subtypes were reported more frequently in patients with NOD2 mutation compared with the control group (Fig. 1). Similarly, in the group of individuals ≥ 50 years old, luminal A (49% vs. 15%) and luminal B HER2(–) (29% vs. 23%) subtypes were reported more often in patients with NOD2 mutation (Fig. 2). The risk of the presence of other BC subtypes than luminal A among NOD2-mutation carriers is lower compared with the control group, independently of patients’ age (Table 3).
Logistic regression analysis results
Factors that were significantly associated with the presence of NOD2 mutation among BC subgroups, in patients aged ≥ 50 years and < 50 years, were detected. Patients ≥ 50 years of age were characterised by lower tumour size (T1–T2), lower histological grade (G1–G2), positive steroid receptor status (ER+ and PR+), and tumours without HER2 overexpression. Conversely, younger (< 50 years) NOD2-mutation carriers were characterised by the deficiency of lymph node metastases (N0), ER+ status, and tumours without HER2 overexpression (Table 3).
Multivariate logistic regression analysis results for NOD2 mutation among patients with BC is shown in Table 4. Among patients with BC at the age ≥ 50 years, the presence of NOD2 mutation was significantly associated with lack of HER2 overexpression (OR = 0.12; p = 0.0001), lower tumour size (T; OR = 0.35; p = 0.041), and ER+ status (OR = 2.03; p = 0.046). However, among patients with BC below the age of 50 years the presence of NOD2 mutation was significantly associated with HER2-negative status (OR = 0.25; p = 0.0001) and insignificantly with ER-positive status (OR = 1.94; p = 0.058).
Table 4
Univariate and multivariate logistic regression
Multivariate analysis has shown, for all study groups, that the older age (OR = 0.61; p = 0.020), ER+ status (OR = 2.10; p = 0.003), and tumours without HER2 overexpression (OR = 0.17; p = 0.0001) were risk factors for NOD2 mutation in patients with BC (Table 4).
NOD2-mutation carriers at the age of < 50 years vs. ≥ 50 years
The comparison of patients with NOD mutations in age groups < 50 and ≥ 50 years indicated a tendency towards higher incidence of negative factors in the group of younger patients with BC (Table 5). BC tumours with G3 (40% vs. 17%; OR = 3.20; p = 0.004) and with HER2 overexpression (22% vs. 10%; OR = 2.62; p = 0.043) were observed significantly more often among younger patients with NOD2 mutation. The tendency towards the presence of larger tumour size (T3–T4) in younger NOD2-mutation carriers was also observed.
Table 5
Pathological characteristics of the tumours in breast cancer patients with NOD2 mutation according to age
Multivariate analysis of pathological factors in the group of individuals with NOD2 mutation has shown that only G3 (OR = 3.22; p = 0.003) was significantly associated with the presence of NOD2 mutation in younger patients.
Discussion
In the present study, NOD2-mutation carriers at age ≥ 50 years were compared with the control group and with the younger (< 50 years) subgroup of patients with NOD2 mutation; according to the clinicopathological factors, such as hormone status ER, PR, HER2, tumour size, the presence of lymph node metastases, and history of cancer in the family.
The insignificant association between the NOD2 3020insC mutation and a family history of BC was reported by Huzarski et al. [5]. The 3020insC mutation of the NOD2/CARD15 gene may also be a genetic predisposing factor for aggregations of breast and lung cancer. Lener et al. reported that the presence of NOD2 mutation was higher in patients with BC who had a first- or second-degree relative diagnosed with lung cancer compared with patients who had no relatives affected by lung cancer [11]. Janiszewska et al. [8] suggested that the NOD2 3020insC mutation may increase the risk of developing gastrointestinal cancer rather than BC. Similarly, in a study conducted by Kurzawski et al., the frequency of the 3020insC mutation in 250 patients with non-hereditary nonpolyposis colorectal cancer at age >50 years was significantly higher in comparison to the control group (OR, 2.23; p = 0.0046) [9]. In another study, the risk of gastric cancer in NOD2 3020insC-mutation carriers at age >50 years was more than doubled (OR = 2.479; p = 0.022) and was almost even three-fold greater among women [10]. In a previous analysis, the presence of NOD2 mutation was associated with an increased risk of a family history of breast, renal, and colorectal cancer [7]. In the present analysis, a family history of cancer, including BC, was observed more often in NOD2-mutation compared with the control group; in patients at age ≥ 50 years. In the group of patients at age < 50 years, the presence of cancer in family history was similar between NOD2-mutation carriers and the control group. Conversely, a family history of colorectal and pancreatic cancer was observed significantly more often in younger NOD2-mutation carriers compared with the control group.
The association between NOD2 mutation and clinicopathological factors in cancer was described only in a few data [7, 12]. Lakatos et al. conducted a study on patients with colorectal cancer and reported no association between clinicopathological factors (such as patient’s age or symptoms at diagnosis) and the CARD15/NOD2 variants carrier status [12]. In a previous analysis, no differences between NOD2 (3020insC) mutation carriers and non-carriers were reported, according to co-morbid condition, drugs, tumour size, steroid receptor status, and five-year overall survival. NOD2 mutation in women with BC was characterised by lymph nodes without metastasis (N0), lower histological grade (G<3), and negative HER2 receptor status (HER2–) [7].
In the present analysis, the subgroup of BC NOD2-mutation carriers at age ≥ 50 years was characterised by lower tumour size (T1–T2), lower histological grade (G1–G2), positive steroid receptor status (ER+ and PR+), and tumours without HER2 overexpression. Patients at age < 50 years are characterised by lymph nodes without metastases, ER+ status, and tumours without HER2 overexpression. There was no difference between NOD2-mutation carriers and the control group observed, according to the BC histological type.
ER+ status and tumours without HER2 overexpression were mostly characteristic of patients with BC with NOD2 mutation, independently of age, in comparison to the control group. Multivariate logistic regression has improved these results and has showed that patients at age ≥ 50 years have a lower risk of NOD2 mutation compared with the younger age group (OR = 0.61; p = 0.020).
In the group of patients with NOD2 mutation at age ≥ 50 years, histological grades G3 and HER overexpression were observed significantly less often compared with younger NOD2-mutation carriers.
Luminal A and luminal B HER2 BC subtypes were most characteristic of patients with NOD2 mutation, independently of age. This analysis was conducted on a larger group of patients, in comparison to the previous study.
The present study has potential limitations. The most important limitation is the group size. Due to some differences between the previous and present studies, a larger group of patients is required. HER2-negative status was characteristic of patients with BC with NOD2 mutation, independently of age, compared with the control group, in the previous and present studies. The other factors such as N0 and G1–G2 were reported from univariate analysis in the present study, but in different subgroups. N0 is observed significantly more often in NOD2-mutation carriers at age < 50 years and G1–G2 in the subgroup of individuals at age ≥ 50 years. In a previous study, patients without NOD2 mutation and NOD2-mutation carriers had similar ages at diagnosis. This can be the cause of the differences.
Conclusions
A family history of BC was characteristic of NOD2-mutation carriers at age ≥ 50 years. HER2 overexpression and ER status were significantly associated with the presence of NOD2 mutation in patients with BC, independently of age. Luminal A or luminal B HER2-negative BC subtypes were most characteristic of patients with NOD2 mutation, independently of age.
In NOD2-mutation carriers at the age ≥ 50 years, the presence of higher tumour size, G3 or HER2 overexpression were lower compared with younger patients. Multivariate analysis has shown that only G significantly differentiates both groups of NOD2-mutation carriers.