Introduction
Cervical cancer is the fourth most common cancer among women worldwide, causing annually about 275,000 deaths [1, 2]. There are many factors affecting the development of this life-threatening disease, such as the socio-economic status, the moment of sexual intercourse, alcohol consumption or smoking, as well as genetic load, immunosuppression and a large number of pregnancies and births (especially for young women) [3]. However, the most important factor that has a huge impact on the development of cervical cancer is primarily persistent infection with high risk HPV (HR HPV), which can lead to an uncontrolled course of infection. It has been demonstrated that HPV is primarily a precursor to the development of cervical carcinogenesis (about 70% of all cancers are caused by the most severe HPV-16 and HPV-18, with 99.7% of cancer cases being detected) [4].
The modern HPV taxonomy is based primarily on the assessment of changes that occur in the evolution of viruses, and not on their characteristic phenotypic traits. This direction of research is influenced by the fact that various, often very phylogenetically distant, HPV types may be associated with the induction of similar symptoms. A perfect example of this phenomenon is HPV-16 and HPV-18, both highly oncogenic types (occurring in pairs or individually), having an indisputable effect on the development of cervical cancer. They are definitely less related to each other than to the types of virus whose presence or impact on the carcinogenesis of this organ has never been confirmed [5].
HPV is transmitted most often through sex, infecting 6.2 million new people each year, which makes it one of the most frequently transmitted viruses in the world. Regardless of gender, and depending on the degree of sexual activity, the risk of HPV infection is around 50% throughout life (both in women and in men) [6]. Women most often get infected with a virus between 15 and 25 years of age. Most infections among immunocompetent women pass asymptomatically after about 24 months. It is worth noting that the lack of symptoms is a hallmark of HPV-induced infection, which undoubtedly contributes to ignoring the patient’s regular tests confirming the presence or absence of pathogen threat, but also allowing the virus to initiate a persistent infection [7].
If the infection does not subside, within 10–30 years of viral replication it will be accompanied by neoplastic changes, concerning the endothelium of the cervix, called CIN (cervical intraepithelial neoplasia). In histopathological findings, they are divided according to the degree of neoplasia from low-grade dysplasia, referred to as CIN-1, medium-sized neoplasia is CIN-2 and the precancerous stage is referred to as CIN-3. In addition, cancer stages are also distinguished, which are classified depending on the stage of cancer disease, i.e. from I to IV [6, 8].
The development of techniques of genetic engineering and molecular biology that took place in the 1980s undoubtedly contributed to the expansion of knowledge about HPV. Thanks to the current polymerase chain reaction (PCR) technique, it has been possible to discover and describe over 200 types of this virus, of which in about 150 cases the isolation and complete sequencing of viral genomes has been made. Moreover, it has been proven that HPV types are molecularly quite divergent, and individual viral genomes evolve at a similarly slow pace as in the genomes of their hosts [5, 9, 10].
In this study we used PCR (Cobas 4800 HPV test) to detect HR HPV DNA in high-risk females from Poland.
Material and methods
Patients
The cervical smear samples were obtained from women treated at the Department of Gynaecology, Institute of Polish Mothers Memorial Hospital in Lodz during the years 2015–2019. The patients’ age ranged from 20 to 56 (mean: 34, SD: 6,7).
HPV DNA detection
Cervical canal swabs (Cobas PCR collection media, Roche Molecular Systems, Inc.) were obtained in 280 patients. The Cobas 4800 HPV test (Roche Diagnostics, GmbH, Mannheim, Germany) – which is a qualitative test device for detection of HPV DNA – was used to analyze the samples. This test amplifies target DNA in cervical epithelium cells (Cobas PCR collection media, Roche Molecular Systems, Inc.) by PCR and nucleic acid hybridization to detect 14 HR HPV types, of which HPV-16 and HPV-18 are of the greatest importance. Moreover, this analysis also enables detection of other HR HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) in clinically significant levels of infectivity.
Sample preparation
Sample preparation was a fully automatized process performed by the Cobas 4800 HPV test device (Roche Diagnostics GmbH, Mannheim, Germany). After collection to the culture medium according to the manufacturer’s manual (Cobas PCR collection media) the samples were digested under denaturing conditions in elevated temperatures and then lysed with a chaotropic agent. Liberated nucleic acids with beta-globin DNA – acting as a process control – were cleaved by absorption to magnetic glass molecules.
PCR amplification
Cobas 4800 HPV test primers were used to amplify DNA of 14 HR HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68). Fluorescent oligonucleotide probes match with the polymorphic region of the primer-determined sequence. The human beta-globulin-oriented fluorescent probe provided quality control of the whole reaction.
Results
Table 1 presents the frequency of HR HPV in the patients. The test revealed 56 samples (20%) to be positive regarding HPV-16 and 40 samples (14.28%) contained HPV-18. Moreover, 94 patients (33.57%) were positive regarding oncogenic HR HPV other than HPV-16 and HPV-18. In 90 (32.14%) patients no HR HPV was detected.
Discussion
Both detection of early lesions and identification of predisposing factors have equally crucial importance in prophylactics of cervical cancer. However, taking into account the current state of knowledge and the current view on the etiology of cervical cancer, it was found that cytological examinations and their evaluation system (used since the 1950s, referred to as the gold standard, the Papanicolaou scale – Pap smear) do not constitute the entire diagnostic set that the patient can use. Currently, it is also recommended to perform diagnostic tests extended with a molecular algorithm. Thanks to it, it is possible not only to detect the HPV virus in the cytological material, but also to determine what specific types of virus are present in it, highlighting above all the most dangerous from the point of view of the implications of the carcinogenesis process. It is also worth paying attention to the limitations of the test related to cytology. This test is characterized by relatively low sensitivity and repeatability, which leads to variable accuracy of the obtained results. In addition, repeating the cytological examination, a greater number of false diagnoses were noted, increasing with time. Moreover, cytology works mainly in the detection of cervical squamous cell carcinoma, in addition to which cervical adenocarcinoma is also present, which unfortunately does not detect this. It is not surprising then that for many years attempts have been made to „support” prophylactic activities concerning cervical research, based mainly on cytology, adding to this standard characterized by significantly higher quality and sensitivity of molecular tests [1, 2, 7].
It is impossible not to notice more clearly the disproportion characterizing the global incidence rate of cervical cancer. It is abnormally higher among women living in undeveloped areas (85% of cases come from these areas), above all Sub-Saharan Africa, Central America and South-East Asia. A clear difference between countries (areas) considered to be developed, and those undeveloped or developing, is explained, among other differences in the risk of HR HPV infection, by the level of consciousness of patients and the system of prevention of this disease. The lowest rates of both morbidity and, above all, mortality are observed in countries where an efficiently operating screening program is available, which results in the morbidity and mortality from cervical cancer falling for years [1, 2].
In the data analysis of a multicentre comparative case study of the International Agency for Research on Cancer (IARC), the odds ratio (OR) for squamous cell carcinoma due to HPV infection was 158.2, with the analysis limited to studies using approved HPV detection techniques [11]. In this study, the odds ratio for cervical cancer ranged from 109 to 276 in studies from different parts of the world [11].
In the prevention of cervical cancer, it is not only important to detect changes early, but also to identify factors that have the most probable etiopathogenetic relationship to the carcinogenesis process within this organ. By detecting and then diagnosing HPV infected people, a highoncology risk group can be identified, which can then be subjected to tighter control. Attempts to estimate the prevalence of HPV infection among women with subclinical or latent disease produce different results, depending on the population studied and the method used to detect the virus. Human viruses from the Papilloma family do not multiply in cell cultures. So far no model of infection of animals and breeding of viruses has been developed. Due to the difficulty of obtaining a viral antigen, standard serological methods cannot be used in in vitro cultures. Detection of HPV infection has become possible only after the introduction of methods used in molecular biology. The highest percentage of infections is diagnosed using a PCR reaction that is characterized by the highest sensitivity among all currently known molecular biology techniques. It allows one to demonstrate the presence of one copy of HPV in 105–106 cells. PCR is now becoming a common diagnostic technique that is used in many laboratories. The results obtained on this basis are comparable and allow one to some extent to avoid their false interpretation. The introduction of DNA testing to detect the presence of HPV virus has increased the effectiveness of screening programs for detection of cervical cancer by prior detection of high-risk changes in women over 30 years of age with the NILM (negative for intraepithelial lesion or malignancy) cytology test and reducing the need for unnecessary colposcopy and treatment in patients after 21 years with the ASC-US result of cytological examination [12–15].
In addition, the sensitivity of the test for the presence of HPV DNA as compared to PAP smear in the detection of a high-grade disease in the population undergoing screening has been repeatedly confirmed. DNA testing for the presence of HPV virus with proven higher sensitivity was proposed and used as the primary first-line screening test in some screening programs.
The Roche Cobas 4800 HPV test (Cobas) is a novel molecular method based on real-time PCR. It has been demonstrated that the Cobas test has good sensitivity, accuracy, and reproducibility for detecting 14 high-risk HPV genotypes [16–18]. The test specifically identifies genotypes 16 and 18 while simultaneously detecting the other high-risk genotypes (pool of 12 genotypes: 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). The ATHENA (Addressing THE Need for Advanced HPV Diagnostics) study, involving more than 47,000 women in the United States, demonstrated that the Cobas 4800 HPV test is clinically validated for ASCUS triage. In summary, the Cobas 4800 HPV test proved to be a useful tool for HPV molecular diagnosis [19].
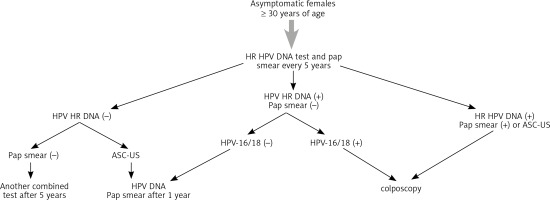
Figure 1 demonstrates the guidelines of the Polish Gynecologic Association Experts Group and the National Chamber of Laboratory Diagnosticians on application of the HPV DNA test in prophylactics of cervical cancer.
Conclusions
The Laboratory of Cancer Genetics, Department of Pathology, Polish Mother’s Memorial Hospital-Research Institute, Lodz, Poland has introduced the above-described methodology (Cobas 4800 HPV test) into routine application in detecting HR HPV infections. The abovementioned results prove that it is a viable, effective, easy and quick tool in detecting high risk HPV DNA and can be introduced into primary screening.