Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is caused by mutations in the polycystic kidney disease 1 (PKD1) or 2 (PKD2) gene and is characterized by formation and enlargement of fluid-filled cysts in the kidneys [1]. Caroli syndrome (CS), also a rare disease, is genetically acquired via a mutation in chromosome 6 (polycystic kidney and hepatic disease 1 (PKHD1) gene), and is characterized by cystic saccular dilatation of fluid-filled intrahepatic ducts [2]. Renal cysts have been reported to rupture, causing a host of complications including hematuria, peritonitis, septic shock and even death [3-6]. By contrast, rupture of cystically dilated intrahepatic ducts has not been reported.
The PCK rat carries a mutation in the PKHD1 gene but phenotypically resembles ADPKD and CS [7, 8]. Kidney and liver images from PCK rats were evaluated to test the hypothesis that the cystically dilated intrahepatic duct wall but not the renal cyst wall undergoes adaptive remodeling.
Material and methods
Image analysis
Archival digitized images from Masson’s trichrome-stained renal and Picrosirius red (PSR)-stained renal and hepatic cross-sections (0.5×, 4× or 10×) were sourced from a drug-naive male PCK rat (7 months) and age-matched male Sprague-Dawley rats (wild-type) from a previous study [8]. Renal cysts (n = 18) were examined from 9 PCK rats; wild-type kidneys were cyst-free. Intrahepatic ducts (n = 26) from 9 wild-type animals were examined; n = 28 intrahepatic ducts were examined from 8 PCK rats. Images were analyzed using ImageJ. Wall thickness was measured at 3 randomly selected but distinct loci along the cyst and duct walls, averaged, and expressed in mm; renal cyst cross-sectional area and intrahepatic duct cross-sectional area were expressed in mm<sup>2</sup>. Circularly polarized PSR microscopy was utilized to observe accumulation of collagen and identify its subtype based on the birefringence pattern [9].
Statistical analysis
Data are expressed as average ± standard error of mean. Between-group differences were calculated using Student’s t-test and a p-value < 0.05 was assumed significant. Correlations between cross-sectional area of the cyst or duct and its wall thickness were made and curve-fit using Microsoft Excel. Renal cyst and intrahepatic duct collagen content was expressed as a percentage of the field area under observation.
Results
Livers from the wild-type cohort exhibited normal intrahepatic ducts (Fig. 1A, B) whereas livers from PCK rats exhibited cystically dilated intrahepatic ducts (Fig. 1A, C). Cross-sectional area of the intrahepatic duct was ~5-fold greater in the PCK cohort vs. the wild-type cohort (Fig. 1D). Nevertheless, duct wall thickness was more pronounced, ~27-fold greater, in the PCK liver compared to the wild-type cohort (Fig. 1E). Thus, the ratio of duct cross-sectional area to wall thickness was reduced in the PCK liver compared to the wild-type liver (Fig. 1F).
Fig. 1
Intrahepatic ducts in wild-type and PCK rats. A) Representative image from PSR-stained wild-type and PCK livers showing hepatomegaly and cystic dilatation of intrahepatic ducts in the latter. B) Intrahepatic ducts in a PSRstained wild-type rat liver enclosed by the duct wall (arrows). C) Cystically dilated intrahepatic ducts in a PSR-stained PCK rat liver showing thick walls. Intrahepatic duct cross-sectional area (D) and wall thickness (E) are increased in the PCK rat compared to the wild-type cohort. F) Ratio of duct cross-sectional area to wall thickness is reduced in the PCK rat

The renal parenchyma of wild-type animals was cyst-free (Fig. 2A, B), whereas PCK rat kidneys were punctuated with numerous cysts enclosed by relatively thin walls (Fig. 2A, C). In fact, the cyst wall was ruptured in one or more places in many of the larger renal cysts (Fig. 2D, E, arrows). Cross-sectional areas and cross-sectional area to wall ratio of intrahepatic ducts (wild-type and PCK) and renal cysts (PCK) were compared. Renal cysts had much larger cross-sectional areas than intrahepatic ducts (Fig. 3A). Equally striking, cross-sectional area to wall ratio of renal cysts was multiple times larger than that of the ducts (Fig. 3B).
Fig. 2
Renal cysts in the PCK rat. A) Representative images from Masson’s trichrome-stained wild-type and PCK kidneys. The renal section from the PCK rat kidney is enlarged and punctuated by cysts. B) Masson’s trichrome-stained renal parenchyma from a wild-type rat is cyst-free. C) Masson’s trichromestained renal parenchyma from a PCK rat is almost completely composed of cysts enclosed by relatively thin walls. D) and E) Large ruptured renal cysts (arrows) in the PCK rat
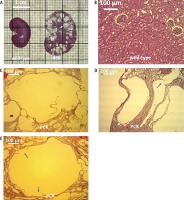
Fig. 3
Duct and cyst characteristics. A) Cross-sectional area of the renal cyst in the PCK rat is multiples times greater than that of the ducts in wild-type and PCK livers. B) Ratio of cross-sectional area to wall thickness is largest in the PCK renal cyst
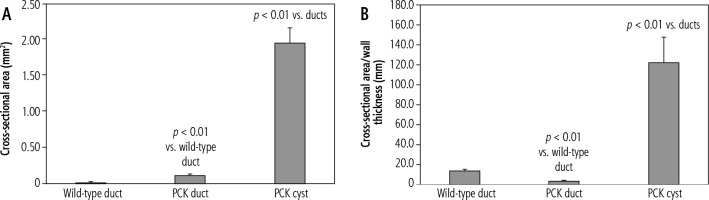
To determine the relation between cross-sectional area of a duct or cyst and its wall thickness, these variables were plotted against each other. Intrahepatic duct wall thickness rose rapidly as a function of duct cross-sectional area and subsequently plateaued, a relation best described by a logarithmic function (Fig. 4A). In renal cysts, increasing cross-sectional area was accompanied by a slowly progressing (slope = 0.0028) increase in wall thickness (Fig. 4B). The linear correlation between these variables in renal cysts was weak but significant (r = 0.49, p = 0.037).
Fig. 4
Duct and cyst wall remodeling. A) As intrahepatic duct cross-sectional area increases, duct wall thickness increases rapidly before plateauing. B) There is a weak but significant direct correlation between renal cyst cross-sectional area and wall thickness
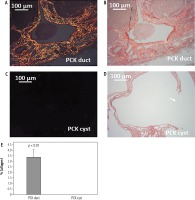
Circular polarized PSR microscopy was used to determine wall composition of intrahepatic ducts and renal cysts. Duct walls appeared a bright yellow-orange (Fig. 5A, B) characteristic of collagen I deposition (Fig. 5E). By contrast, no collagen deposition was evident along renal cyst walls (Fig. 5C-E).
Fig. 5
Collagen I-driven duct wall remodeling. A, B) Circular polarized PSR view and bright-field view, respectively, of PCK rat intrahepatic ducts, exhibiting bright orange-yellow along the duct wall. C, D) Circular polarized PSR view and bright-field view, respectively, of a large renal cyst with a ruptured wall (arrow) in the PCK rat. No collagen birefringence is visible. E) Collagen I (% field) expression in PCK intrahepatic ducts and renal cysts
Discussion
The PCK rat model of ADPKD-CS is characterized by the presence of multiple renal cysts and cystically dilated intrahepatic ducts. Compared to the cyst wall, the duct wall is relatively thick. Intrahepatic duct wall thickness increases rapidly as a function of increasing cross-sectional area and is driven by accumulation of collagen I. By contrast, renal cyst wall thickness exhibits a weak correlation with cross-sectional area with no collagen accumulation.
Adult ADPKD patients are at risk of hemorrhage, peritonitis, sepsis and even death from rupture of enlarged fluid-filled renal cysts. By contrast, there are no reports of rupture of cystically dilated intrahepatic ducts in CS. The present study conducted in the PCK rat model of ADPKD-CS was designed to test the hypothesis that cystically dilated intrahepatic ducts but not renal cysts undergo adaptive wall remodeling. Compared to age- and gender-matched wild-type rat livers, intrahepatic ducts in the PCK cohort had much larger cross-sectional areas. Nevertheless, the increase in duct cross-sectional area was accompanied by a rapid increase in duct wall thickness. As a result, the cross-sectional area to wall thickness ratio was smaller in PKC rat ducts compared to the wild-type cohort. Interestingly, this rapid increase in duct wall thickness plateaued, suggesting that adequate structural support had been achieved. While wild-type kidneys had no cysts, the PKC rat exhibited multiple small and large cysts consistent with the ADPKD phenotype. Renal cysts tended to be larger than the intrahepatic ducts in the PCK rat. Nevertheless, relative to cross-sectional area, cyst walls were significantly thinner than their ductal counterparts. Furthermore, as cyst cross-sectional area increased, wall thickness increased only gradually, evidenced by the shallow slope of this relationship. Interestingly, many of the larger cysts were ruptured in one or more places, whereas none of the intrahepatic ducts were ruptured. Consistent with, and as a consequence of Laplace’s law of wall tension [10], it is possible that the renal cyst wall cannot tolerate the tension experienced by it, causing it to rupture.
Collagen is a major structural component of cellular organelles. The PSR stain is one of the best understood histochemical techniques able to selectively highlight collagen networks. Relatively inexpensive, the technique relies on the birefringent properties of collagen molecules. While the PSR stain alone does not selectively bind the collagen network, it becomes more specific than the other common collagen stains when combined with polarized light detection [9, 11]. Under circularly polarized PSR microscopy, thin bundles of collagen type III appear green-yellow, whereas large bundles of tightly packed, well-organized and mature collagen type I molecules appear yellow-orange [9]. Use of circularly polarized PSR microscopy revealed bundles of yellow-orange collagen I [9, 11] along the intrahepatic duct walls, indicating the presence of mature collagen type I, which provides both structural support and tensile strength [9]. By contrast, there was no accumulation of collagen I or collagen III along the renal cyst walls. Together these data suggest that collagen I-driven remodeling of their walls stabilizes cystically dilated intrahepatic ducts, a mechanism that appears absent in renal cysts.
In as much as the PCK rat is a model of ADPKD-CS, these findings suggest that renal cysts are at increased risk for rupture, whereas cystically intrahepatic ducts do not, as the latter undergo remodeling to accommodate increased wall tension. Needless to say, this finding remains to be confirmed in human subjects. Nevertheless, these data suggest that ADPKD patients at risk for rupture of renal cysts may benefit from therapies including cystocentesis or aspiration of fluid from the cysts, especially the larger cysts, and/or sclerotherapy [12, 13] to relieve wall tension.






