Summary
In 5 patients with no-option critical limb ischemia we performed a first-in-man study to evaluate the safety and feasibility of using Wharton’s jelly mesenchymal stem/stromal cells (WJMSCs) in patients with N-O CLI. WJMSCs were administered (3–6 administrations) at 3–6-week intervals. The study showed the safety and feasibility of our novel therapeutic approach, and suggested treatment efficacy with ≥ 3 doses. Our findings provide a basis for a randomized, double-blind clinical trial to assess the efficacy of the WJMSCs-based therapeutic strategy in N-O CLI patients.
Introduction
Critical limb ischemia (CLI) is an advanced stage of lower extremity arterial disease (LEAD) with presence of ischemic rest pain, ulceration or gangrene. It is associated with high amputation and mortality rates, constituting a major societal problem [1, 2]. Open surgical, endovascular and hybrid revascularization combined with medical treatment remain the standard of care; this present standard, however, is in many patients insufficient. Patients with CLI in whom effective revascularization is not (or no longer) possible and best medical therapy is inefficient are defined as no-option CLI (N-O CLI) patients. In this group, novel effective strategies to promote vascular reparation and neovascularization are critically needed to prevent major amputations [3–5]. Stem cell-based reparative and regenerative therapies have been gaining increasing attention in the last decade. Several different cell lines have been used as potential therapeutic agents in the attempt to modify the course of CLI. Mesenchymal stem/stromal cells (MSCs) may be more effective than other cell types such as hematopoietic cells [6]. Recent pre-clinical evidence showed high regenerative potential of Wharton’s jelly mesenchymal stem cells (WJMSCs) in treating hind limb ischemia [7]. Specifically, in a mouse model of hind limb ischemia induced with femoral artery ligation, the tissue blood flow improved significantly in the WJMSCs-treated animals compared with controls. Furthermore, the histological analysis of ischemic muscles showed visible tissue regeneration, with new proliferating cells, in the WJMSC-treated group. Moreover, the ischemic hind-limb motor function was significantly improved in WJMSC-treated animals compared with controls [7]. The feasibility and procedural safety of myocardial (transcoronary) WJMSC administration as a potential therapeutic agent after acute myocardial infarction have already been demonstrated [8]. However, to date WJMSCs have not been tested in N-O CLI in man.
Aim
This work is a safety and feasibility study of WJMSCs hybrid administration (intramuscular, intra-arterial) as a reparative/regenerative strategy in patients with N-O CLI.
Material and methods
Patient enrollment
This safety and feasibility study aimed to enroll 5 patients. The inclusion criteria were as follows:
Patients with critical lower limb ischemia – presenting with ischemic rest pain, ischemic ulceration or gangrene (stages 4–6 according to the Rutherford classification, and 3–4 according to the Fontaine classification) not feasible for (further) endovascular revascularization due to anatomical and/or technical reasons.
Age ≤ 80 years.
Patent common femoral artery (CFA) with adequate inflow and at least 1 patent below-the-knee artery (evaluated in duplex Doppler ultrasound, angiography or computed tomography angiography)
The exclusion criteria were as follows: history of malignancy, female in reproductive age without effective contraception, breast feeding, local infection in area of puncture sites for assessed product administration, diagnosed immunodeficiency or history of any other clinical condition associated with immunodeficiency, participation in other clinical trials.
All subjects provided informed written consent. The study was approved by the local Ethics Committee and compliant with the Declaration of Helsinki.
Human WJMSCs’ harvest, expansion and preparation for human application
The cells were obtained, harvested and prepared for administration using previously published methods and protocols [8]. In brief, MSCs were isolated from donated umbilical cords using an enzymatic digestion strategy. Isolated cells were plated into 75 cm3 tissue culture flasks in Dulbecco’s modified Eagle’s medium, supplemented with 10% phosphate-buffered saline, PDGF and EGF growth factors. Flasks were incubated at 37°C in a humidified atmosphere containing 5% CO2. After 7 days the medium was changed. At confluence, the adherent cells were detached using 0.25% trypsin and re-seeded at 0.075 × 106 cells/75 cm3 and incubated again until confluence with a weekly medium change. After the second passage, cells were collected and frozen in liquid nitrogen. Five to seven days before administration cells were thawed and expanded to obtain at least 30 × 106 cells [8]. The cell phenotype was analyzed with antibodies specific for MSCs (CD73, CD90, CD105) and hematopoietic cells (CD45). WJMSCs ≥ 95% positive for CD73, CD90, CD105 and negative for CD45 were approved for administration. Cytogenetic stability of cultured WJMSCs was confirmed via Giemsa banding. Metaphases were analyzed under an Olympus BX51 microscope with a camera to document photomicrographs. The CytoVision program was used to arrange chromosomes into a karyogram. Sterility of the investigated medical product (IMP) was confirmed via microbiological evaluation.
Baseline clinical evaluation and follow-up
Before the first administration of the IMP, in each patient past medical history was analyzed (especially concerning previous revascularizations and comorbidities). Based on angiography, computed tomography angiogram and/or duplex/triplex ultrasound, the level of artery occlusion was identified. Clinical stage of limb ischemia was assessed using the Rutherford classification. Safety and potential signals of efficacy were assessed after each IMP administration, prior to any further administration at any point the patient requested contact with the study team, and at 6, 12 and 48 months. Clinical vigilance was applied for any adverse events during the procedure of WJMSC administration and up to 48 months after. At 12 ± 2 months and 48 ± 2 months after the first administration during the follow-up visits, the clinical condition of the ischemic limb was re-assessed using the Rutherford classification. Presence of ischemic rest pain, claudication distance, necrosis progression or ulcer healing was also evaluated. Major amputation and/or death (if it occurred) was to be reported at any time during follow-up.
WJMSCs administration
Combined intra-arterial and intra-muscular (50%/50%) IMP administration at 6-week intervals was performed. A total of 3–6 doses per patient were delivered in order to assess the safety and efficacy signals of different total dosage regimens, in preparation for a potential endpoint-powered randomized study.
With each IMP administration, 30 × 106 WJMSCs suspended in 30 ml of 0.9% saline were injected. 15 ml of suspension was injected intra-arterially (into the common femoral artery, under ultrasound guidance using a standard 20 ml syringe and 22G needle). Another 15 ml was administered in 3 intramuscular injections into the gastrocnemius muscle and 2 injections into the quadriceps muscle (3 ml each). All procedures were performed during out-patient visits. Manual compression was applied on the puncture site directly after intra-arterial injection and continued for 10 min, with subsequent hemostasis evaluation and application of non-compressive sterile dressing. After intramuscular injections, all puncture sites were covered with sterile dressings and a 1st class compression bandage was applied on the limb for 15 min to prevent bleeding and hematoma formation.
Results
Clinical characteristics of the study group, number of WJMSC administrations and time intervals are presented in Table I. Five patients (age 61–71, 60% male, Rutherford-6 20%, Rutherford-5 60%, Rutherford-4 20%) were enrolled. Detailed patient characteristics on enrollment and at 12 months and 48 months of follow-up are presented in Table II.
Table I
Study group initial clinical characteristics, number of WJMSCs administrations and time interval between them
Table II
Detailed patient characteristics before and after WJMSCs administration
In 1 (20%) of the enrolled patients, study inclusion occurred on a compassionate basis (extensive necrosis, Rutherford 6, pre-amputation stage). Four (80%) patients were enrolled with minor necrosis and/or ischemic rest pain (Rutherford 4–5). One patient (temporary residency abroad) presented to follow-up visits outside of the time window.
In all cases WJMSC harvesting was performed according to the protocol established in the Cell and Tissue Bank providing IMP for use in the study (Jagiellonian University in Krakow, Department of Transplantation). No WJMSCs genetic instability was detected and no other laboratory-safety level issues occurred. In all study patients WJMSCs were administered per protocol in absence of administration technique-related adverse events.
No procedural adverse events concerning WJMSCs administration were observed. In 3 out of 5 patients (60%) after WJMSCs administrations, transient edema, warming and pain increase of the calf (responsive to paracetamol) were observed directly after injection. These resolved spontaneously without sequelae in all patients during the first 48 h after the procedure. Care was taken to note all potential adverse events, and those were reported as per good clinical practice. Rash, warming and transient pain aggravation were considered as probably related to WJMSCs administration.
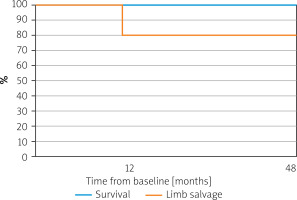
No deaths occurred at 12- and 48-month follow-ups. Amputation-free survival was 80% after 12 and 48 months (Figure 1). A major amputation was performed in 1 (20%) patient enrolled with initial forefoot gangrene (Rutherford 6), 7 days after the third IMP administration due to continued progression of necrosis that was not halted with IMP administration. Among other participants (with a preserved limb) no ulceration or rest pain was observed at 12 and 48 months. Mean claudication distance of 190 ±181.9 (80–400) m was reported at 12 months and 106.7 ±80.83 (60–200) m at 48 months. This was considered an important finding, as prior to exposure to IMP, the walking distance was minimal (rest pain).
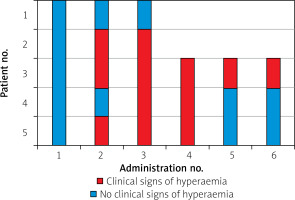
Clinical signs of hyperemia (such as skin reddening and local temperature rise of the limb), lasting 12–24 h, occurred in 3/5 subjects (60%) after the second IMP administration (in no patient after the 1st one), and in 4/5 subjects (80%) after the third IMP injection (Figure 2). However, their occurrence was less common after the 5th and 6th administrations of the IMP (Figure 2).
Discussion
The CIRCULATE N-O CLI Pilot Cohort Study, the first-in-human experience with WJMSCs’ hybrid (combined intra-arterial and intra-muscular) administration, showed the feasibility and procedural safety of WJMSCs transplantation in patients with NO-CLI according to the initial protocol. Moreover, the hyperemic response and improved limb function in the majority of patients (80%) indicated potential efficacy of WJMSCs in N-O CLI.
To date, available controlled trials and meta-analyses have suggested that regenerative therapies may be beneficial in N-O CLI, but no definitive statement can be made [9].
Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) [10] was a randomized, double blinded placebo-controlled trial with the largest number of enrolled individuals (160) among the CLI randomized controlled trials (RCTs) published to date [3, 9, 11]. In essence, this trial provided level-1 data that intra-arterial infusion of BM-MNCs is unlikely to affect the course of CLI.
The RESTORE-CLI [12] Trial assessed the efficacy and safety of intra-muscular injections of ixmyelocel-T – a patient-specific multicellular product derived from autologous bone marrow (biopsy) and produced in an automated closed-culture system. Efficacy assessment included time to first occurrence of treatment failure (TTF, including major amputation, all-cause mortality, doubling of total wound surface area from baseline, de novo gangrene) and amputation-free survival (AFS). TTF was significantly extended for patients treated with the cellular product when compared with controls (p = 0.0032). Furthermore, there was non-significantly higher amputation-free survival in the ixmyelocel-T treated patients compared with the placebo group.
Procházka et al. [13] conducted a trial assessing the efficacy of local intra-muscular application of bone marrow concentrate in treatment of ischemic foot ulcers in 96 N-O CLI patients. In this trial, intra-muscular administration of bone marrow concentrate appeared to improve limb salvage.
Abdul Wahid et al. [3] provided a review on NO-CLI therapies based on autologous cells derived from different sources and administered using different regimens. They included seven RCTs with a total of 359 participants. Not only did the authors try to assess the “global” efficacy of cellular therapies in NO-CLI but they also compared bone marrow mononuclear cells (BM-MNCs) vs. mobilized peripheral blood stem cells (mPBSCs), BM-MNCs vs. bone marrow- mesenchymal stem cells (BM-MSCs), high versus low doses, and routes of product administration – intra-arterial (IA) vs. intra-muscular (IM). Overall, no clear differences between different stem cell sources and treatment regimens were found for the outcomes all-cause mortality, amputation rate, ulcer healing, and rest pain (with mostly low or very low quality of evidence). Similarly, no clear difference in efficacy was found between IA and IM administration. No significant short-term adverse effects were reported. As a general conclusion, the authors stated that high-quality evidence is lacking to confirm the efficacy and long-term safety of autologous cell transplantation in NO-CLI.
An integrated review of pre-clinical and clinical studies by Qadura et al. [14] concluded that, despite promising preclinical studies in animal models, transplantation of bone marrow derived cells in NO-CLI patients shows limited benefits.
A meta-analysis published by Gao et al. [11] in 2019 that included 27 RCTs (with a total of 1186 patients) showed that autologous stem cell therapy had a greater effect than conventional therapy on the ulcer healing rate, and significantly improved ABI, TcO2 and pain-free walking distance. The researchers concluded that NO-CLI patients “may benefit from stem cell therapy,” but larger, randomized, double-blinded, placebo-controlled, multicenter trials with long-term follow-up are still needed.
The majority of the above-mentioned studies concerning treatment of critical limb ischemia using stem and progenitor cells focused on use of autologous bone marrow (BM-MSCs) and peripheral blood-derived mesenchymal stem cells (PB-MSCs) [3, 11]. To the authors’ best knowledge, to date there are no completed clinical trials providing data on safety and efficacy of WJMSCs-based therapies in NO-CLI. Meanwhile this cell population seems to be particularly attractive for regenerative therapy in cardiovascular diseases. WJMSCs express all surface antigens typical for MSC, are easy to isolate (without an invasive procedure as in the case of BM-MSCs) and harvest without ethical concerns [7, 8, 10, 11, 12, 15]. WJMSCs spontaneously secrete pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), angiopoietin-1, transforming growth factor β1 (TGF-β1) and hepatocyte growth factor (HGF) [16]. Because WJMSCs do not express major histocompatibility complex class II (HLA-DR) antigens or surface antigens CD40, CD80, CD86, they do not elicit an allogenic immune response or transplant rejection [14]. WJMSCs possess stemness properties that last several passages in vitro and are multipotent, but do not induce tumorigenesis, even though they have some embryonic stem cell markers [17, 18]. Furthermore, expansion of WJMSCs is not associated with loss of genetic stability [19] (as confirmed in our laboratory evaluation), consistent with a high index of safety of a WJMSCs-based IMP. The features listed above encourage efforts to create regenerative therapy for NO-CLI based on an “off-the-shelf” WJMSCs product [8].
IMP post-administration hyperemia as a potential signal of efficacy
Transient fever and rash after administration of cellular therapeutic agents (both autologous and allogenic) have been reported [8, 17–21]. After transcoronary WJMSCs administration, a paracetamol-sensitive temperature rise occurred in 1 patient (resolution without sequelae) [8]. In our cohort we did not observe fever after WJMSCs injection, while multiple cases of transient rash, warming and increased pain of the treated limb occurred (Figure 2). A similar “adverse” event was reported in the literature after administration of BM-MNCs in the RESTORE-CLI Trial [12]. Although the mechanism of this event has not yet been described, we hypothesize that it may be a tissue reaction to vasoactive cytokines released by WJMSCs, as it is clinically similar to the reaction frequently observed after therapeutic prostaglandin E1 injection. We believe that transient hyperemia of the treated limb can be considered as a cytokine-related effect of the IMP and should be treated as a signal of short-term efficacy rather than an adverse event. The fact that signs of hyperemia in our cohort were most commonly observed after the 3rd and 4th WJMSCs deliveries may suggest that cytokine-dependent vasodilation was more likely induced with multiple rather than a single dose of IMP. In future studies multiple administrations of therapeutic IMPs should be therefore considered to enhance the hypothesized therapeutic potential.
No association between MSCs injection and acute infusional toxicity, organ system complications, infections, death or malignancy was reported [19].
Patient selection for N-O CLI trials
The most common cause of CLI is LEAD. Patients affected with CLI, especially those insusceptible for further revascularization procedures, are characterized by high comorbidity (particularly diabetes, coronary artery disease, and chronic cardiac failure). It has been estimated that 25% of patients diagnosed with CLI will die within 12 months from diagnosis, and an additional 30% will undergo major limb amputation [1, 2]. Thus, recruitment of patients with CLI to clinical trials and sustaining them for follow-up is challenging even though morbidity from peripheral artery disease is high and is increasing [22]. As a consequence, the evidence level on regenerative strategies in CLI remains low. Data from the literature suggest that cell therapies may be beneficial in patients without extensive gangrene, and inefficient in individuals classified as Rutherford 6 (who have crossed the “point of no return”) [23]. Also in our cohort, the only patient who underwent major amputation after IMP administration was one initially assessed as Rutherford 6.
Limitations
The number of evaluated participants can be considered as a limitation of this study; our sample size, however, is consistent with the naturally modest size of FIM studies. As the primary aim of this study was to define the safety and feasibility of WJMSCs administration in NO-CLI patients, evaluation of therapy efficacy was based, at the present point, on clinical findings. In future trials more advanced, objective methods assessing limb perfusion are required.
Conclusions and future directions
This study demonstrated the feasibility and procedural safety of WJMSCs product administration in N-O CLI. Randomized, placebo-controlled, blinded trials are required to evaluate the efficacy of this regenerative strategy. Repetitive (≥ 3 doses) instead of single dosage of stem/stromal cell administration should be considered in future trials as well as a combined (intra-arterial and intra-muscular) route of delivery to enhance the therapeutic potential.