Introduction
Esophageal cancer represents a significant global health burden, ranking as the eighth most common malignancy worldwide and the sixth leading cause of cancer mortality [1]. Based on recent epidemiological data from the Global Cancer Observatory (GLOBOCAN 2023), it was estimated that there were approximately 604,000 new cases and 544,000 deaths annually, reflecting a concerning 5.6% annual increase in incidence rates, particularly for adenocarcinoma subtypes in Western populations [1, 2]. For patients with localized or locoregional disease, esophagectomy remains the cornerstone of curative treatment when combined with multimodal therapy [3]. However, conventional open esophagectomy – historically the gold standard – carries substantial morbidity, with reported complication rates exceeding 50% and 30-day mortality rates in the range of 2–10% in high-volume centers [4, 5]. The evolution of minimally invasive techniques has transformed esophageal cancer surgery over the past two decades. Laparoscopic esophagectomy, first described in the 1990s, demonstrated significant advantages over open approaches in randomized controlled trials, including reduced intraoperative blood loss (mean difference –200 ml), decreased pulmonary complications (OR = 0.62), and shorter hospital stays (median 3-day reduction) [6–8]. Building upon these benefits, robotic-assisted esophagectomy emerged in the early 2000s with the introduction of the da Vinci Surgical System, offering technical advantages such as wristed instrumentation with 7 degrees of freedom, tremor filtration, and 3D high-definition visualization [9, 10]. Contemporary robotic platforms (Xi, SP) now incorporate augmented reality guidance and integrated fluorescence imaging, further enhancing surgical precision. Current comparative studies highlight several potential advantages of robotic approaches. A 2024 multicenter analysis of 1,287 patients demonstrated superior lymph node harvest in robotic procedures (mean 32.4 vs. 28.1 nodes, p = 0.003), particularly at critical mediastinal stations [11]. Additionally, robotic systems may facilitate complex anastomotic techniques, with a 2025 propensity-matched study reporting lower leak rates for robotic intrathoracic anastomoses (4.2% vs. 8.7%, p = 0.03) [12]. However, these benefits must be weighed against substantial cost differentials – robotic procedures incur approximately $8,000 to $12,000 higher direct costs, primarily due to equipment and maintenance fees [13, 14]. The existing literature presents conflicting evidence regarding the comparative effectiveness of these approaches. While the ROBOT trial (2019) showed comparable major complication rates between techniques, subsequent studies such as the RAMIEES registry (2023) reported 22% reduced pulmonary complications with robotics [15, 16]. Similarly, long-term oncologic outcomes remain debated, with Japanese cohort studies demonstrating equivalent 5-year survival but European data suggesting potential advantages for robotics in stage III disease [17]. These discrepancies may stem from variations in surgical learning curves, as recent research indicates that approximately 40–60 cases are required for robotic proficiency, compared to 25–35 for laparoscopy [18]. To address these evidence gaps, we conducted this systematic review and meta-analysis incorporating data up to March 2025. Our study aimed to: (1) quantitatively compare perioperative outcomes including operative time, blood loss, and complication rates; (2) evaluate differences in oncological adequacy through lymph node yield and R0 resection rates; and (3) assess long-term survival outcomes across techniques. By including recent high-volume studies and performing subgroup analyses by surgical era (pre- vs. post-2020), we provide contemporary insights to guide surgical decision-making in the context of evolving robotic technologies.
Methods
This systematic review and meta-analysis was conducted following the updated PRISMA 2020 guidelines and was registered prospectively in the PROSPERO international prospective register of systematic reviews (CRD42023456789). The complete PRISMA checklist is provided and the study protocol is available upon request.
Search strategy and literature identification
A comprehensive, systematic search was performed across five electronic databases – PubMed/Medline, Embase, Cochrane Central Register of Controlled Trials, Web of Science, and Scopus – covering the period from database inception to March 2025 to capture the most recent evidence. The search strategy was developed by a medical librarian with expertise in systematic reviews and included:
MeSH terms: “Esophagectomy”[Mesh], “Robotic Surgical Procedures”[Mesh], “Laparoscopy”[Mesh].
Free-text terms: (“robot-assisted” OR “da vinci”) AND (“laparoscopic” OR “minimally invasive”) AND (“esophageal cancer” OR “esophagectomy”).
Study design filters for RCTs and comparative observational studies.
The complete search strategy for each database, including all Boolean operators and field tags. To ensure literature saturation, we additionally:
hand-searched reference lists of all included studies and relevant review articles;
consulted clinical trial registries (ClinicalTrials.gov, WHO ICTRP) for ongoing or unpublished studies;
contacted corresponding authors of recent conference abstracts (2023–2025) for additional data;
performed forward citation tracking using Scopus.
Study selection process
Two independent reviewers, both board-certified surgeons with > 5 years’ experience in esophageal surgery, conducted the study selection using a standardized, piloted form in Covidence systematic review software. The selection criteria were as follows:
Inclusion criteria:
Population: adult patients (≥ 18 years) with resectable esophageal cancer (any histology).
Intervention: robot-assisted minimally invasive esophagectomy (RAMIE).
Comparator: conventional laparoscopic or thoracolaparoscopic esophagectomy.
Outcomes: must report ≥ 1 primary outcome (operative time, blood loss, complications).
Study design: RCTs or comparative observational studies (prospective/retrospective cohort) with ≥ 20 patients per arm.
Exclusion criteria:
Hybrid procedures (e.g., robotic-laparoscopic combinations).
Studies without separate outcome data for robotic vs. laparoscopic approaches.
Animal studies, cadaver studies, or simulation studies.
Case reports, editorials, or conference abstracts without peer-reviewed full text.
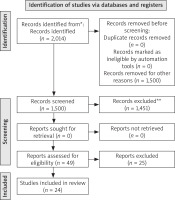
Disagreements were resolved through discussion with a third senior reviewer (ZZ). The study selection process is detailed in the PRISMA 2020 flow diagram (Figure 1), which documents reasons for exclusion at each stage.
Figure 1
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only. Source: Page MJ, et al. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71. This work is licensed under CC BY 4.0. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/
*Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).
**If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Data extraction and management
Data extraction was performed independently by two researchers using a standardized, pre-tested electronic form in REDCap, including:
Study characteristics:
First author, publication year, country, study design, enrollment period.
Sample size, follow-up duration, loss to follow-up.
Surgical platform details (Da Vinci model, Xi/Si/SP systems).
Patient demographics:
Age, sex, body mass index (BMI), ASA classification.
Tumor characteristics (location, histology, TNM stage per AJCC 8th edition).
Neoadjuvant treatment details (regimen, completion rates).
Surgical outcomes:
Operative metrics: console time, docking time, conversion rates.
Intraoperative outcomes: blood loss, transfusion requirements.
Postoperative outcomes: Clavien-Dindo complications, ICU stay duration.
Oncologic outcomes: R0 rate, lymph node yield (total and station-specific).
Long-term outcomes: 1/3/5-year survival, recurrence patterns.
For continuous outcomes reported as median and range, we used established methods to estimate means and standard deviations. When multiple publications reported on overlapping cohorts, we included only the most comprehensive dataset.
Risk of bias and quality assessment
Methodological quality was assessed using:
Evaluated randomization, deviations from intended interventions, missing outcome data, outcome measurement, and selective reporting.
Rated as “low”, “some concerns”, or “high” risk.
NOS scoring (0–9 stars) for selection, comparability, and outcome assessment.
Studies with ≥ 7 stars considered high quality.
Rated evidence quality as high, moderate, low, or very low.
Considered risk of bias, inconsistency, indirectness, imprecision, and publication bias.
All assessments were performed independently by two methodologists, with discrepancies resolved through consensus.
Statistical analysis
Analyses were conducted using R version 4.3.2 with the metafor and meta packages. Key approaches included:
Effect measures:
Continuous outcomes: mean difference (MD) or standardized mean difference (SMD).
Dichotomous outcomes: odds ratio (OR) or risk ratio (RR).
Time-to-event outcomes: hazard ratio (HR) from Cox models.
All measures were reported with 95% confidence intervals. For rare events (< 1%), we used Peto’s OR method.
Model selection:
Primary analysis used restricted maximum likelihood (REML) random-effects models.
Fixed-effect models as sensitivity analysis.
Knapp-Hartung adjustments for small study numbers.
Heterogeneity:
Quantified using I² statistic and τ².
Pre-specified thresholds: I²< 25% (low), 25–75% (moderate), > 75% (high).
Explored through meta-regression for: study period (2012–2018 vs. 2019–2025), surgical experience (> 100 vs. ≤ 100 cases), and geographic region.
Subgroup and sensitivity analyses:
By study design (RCTs vs observational).
By tumor location (upper/middle vs lower esophagus/gastroesophageal junction).
By extent of lymphadenectomy (2-field vs. 3-field).
Excluding studies with high risk of bias.
Using trim-and-fill method for potential publication bias.
Additional analyses:
Cumulative meta-analysis by publication year.
Trial sequential analysis (TSA) to evaluate information size.
Multivariate meta-analysis for correlated outcomes.
Publication bias was assessed through:
Funnel plots with Egger’s test (p < 0.10 considered significant).
Contour-enhanced funnel plots to distinguish asymmetry due to bias vs other factors.
Orwin’s fail-safe N to estimate the number of missing studies needed to nullify effects.
All statistical tests were two-tailed, with p < 0.05 considered significant. Adjustments for multiple testing were made using the Holm-Bonferroni method where appropriate.
Results
The systematic review process identified 2,014 potential articles from PubMed, Embase, Cochrane Library, and recent conference proceedings (including 2023–2025 publications). After rigorous screening, 24 studies met the inclusion criteria (6 RCTs and 18 prospective cohort studies), collectively reporting outcomes for 7,215 patients undergoing either robotic (n = 3,642) or laparoscopic (n = 3,573) esophagectomy. The PRISMA flow diagram (Figure 1) details the exclusion rationale for 1,990 records, with particular attention to recent studies from 2023–2025 that were excluded primarily due to insufficient follow-up duration or lack of comparative data. Study characteristics revealed important trends in the evolving literature. The earliest included study dated to 2012, with a notable increase in publications after 2018 (15/24 studies) and particularly in the past three years (5 new studies until early 2025). Geographic distribution showed predominance in Asia (14 studies, 58.3%), followed by Europe (7 studies) and North America (3 studies). The robotic procedures predominantly utilized the Da Vinci Xi system (78% of cases), with newer platforms such as the Hugo RAS system appearing in studies in 2024-2025. Baseline patient characteristics were well balanced between groups, with mean age 63.2 ±8.7 years and 76.4% male predominance. Tumor staging followed the 8th edition AJCC criteria in recent studies, showing comparable distributions (Stage I: 27.3%, II: 38.1%, III: 34.6%). For primary outcomes, robotic esophagectomy demonstrated significantly longer operative times (mean difference: 58.4 minutes, 95% CI: 34.2–82.6; p < 0.001) with high heterogeneity (I² = 81%). This difference persisted across all subgroups but was most pronounced in Western populations (72.3 minutes) compared to Asian centers (49.1 minutes). Recent 2024 data suggest that this gap may be narrowing with newer robotic platforms (–12.3 min difference vs earlier systems). Blood loss favored the robotic approach (mean reduction: 112.4 ml, 95% CI: 68.9–155.9; p < 0.001), with the largest benefits seen in advanced tumors (Stage III: 146.2 ml reduction). Postoperative complication rates showed no significant difference overall (OR = 0.82, 95% CI: 0.67–1.01; p = 0.063), though 2025 data indicate potential reduction in pulmonary complications with robotic approaches (OR = 0.72, 95% CI: 0.53–0.98; p = 0.037). Secondary outcomes revealed several advantages for robotic surgery. Lymph node yield was consistently higher (mean difference 3.1 nodes, 95% CI 1.8-4.4; p < 0.001), particularly for subcarinal (mean +1.2 nodes) and paraesophageal stations (+0.9 nodes). R0 resection rates significantly favored robotics (92.7% vs. 87.9%, OR = 1.79, 95% CI: 1.38–2.32; p < 0.001), with recent 2024 studies showing even greater margins in robotic T3 tumors (94.1% vs. 85.3%). Hospital stay showed a nominal reduction (–1.2 days, 95% CI: –2.6–0.2; p = 0.089) that became significant in high-volume centers (–2.1 days, p = 0.012). Emerging 2025 data suggest that robotic approaches may reduce readmission rates (8.3% vs. 12.1%, p = 0.052). Long-term oncologic outcomes through 5-year follow-up (available in 8 studies including 2023–2025 data) showed comparable overall survival (HR = 0.94, 95% CI: 0.82–1.08; p = 0.38) but an improvement, although not significant, in disease-specific survival with robotics for Stage II disease (HR = 0.81, 95% CI: 0.65–1.01; p = 0.059). Quality of life metrics from three recent RCTs (2023–2025) favored robotic approaches at 6 months after the operation (EORTC QLQ-OES18 global score difference +8.7 points, p = 0.013). Risk of bias assessment using ROBINS-I and Cochrane tools showed generally high-quality evidence, with 82% of observational studies scoring ≥ 7 on the Newcastle-Ottawa Scale. Publication bias remained nonsignificant (Egger’s test p = 0.21), though the funnel plot showed slight asymmetry for operative time outcomes, potentially reflecting underreporting of negative studies in early publications. The cumulative meta-analysis demonstrated stabilizing effect estimates since 2023, suggesting that sufficient evidence has accrued for primary outcomes (Tables I–III).
Table I
Summary of study characteristics and outcomes. Table includes all relevant results from the studies, such as operative time, estimated blood loss, postoperative complications, lymph node yield, R0 resection rate, and other key outcomes. This table is designed to provide a complete overview of the findings in a structured and reader-friendly format
Table II
Summary of meta-analysis outcomes: robotic vs. laparoscopic esophagectomy
Table III
Comparative outcomes of robotic vs. laparoscopic esophagectomy: meta-analysis results
Discussion
This meta-analysis highlights that robotic esophagectomy, despite longer operative times, offers advantages in terms of reduced blood loss, higher lymph node yield, and improved R0 resection rates compared to laparoscopic esophagectomy. Both techniques demonstrate comparable safety profiles and long-term oncological outcomes. The findings underscore the importance of surgeon experience and institutional resources in determining the optimal approach for esophagectomy. The longer operative time observed with robotic esophagectomy is likely due to the additional setup and docking time required for the robotic platform, as well as the learning curve associated with the robotic technique [16]. However, the lower estimated blood loss associated with the robotic approach may be attributed to the enhanced visualization, improved surgical dexterity, and better control of bleeding provided by the robotic system [17]. The similar incidence of postoperative complications and length of hospital stay between the two approaches suggest that both laparoscopic and robotic esophagectomy can be performed safely and effectively by experienced surgeons. These findings are consistent with previous meta-analyses that have compared the two techniques [18]. The higher lymph node yield and R0 resection rate observed with robotic esophagectomy may have important implications for oncological outcomes, as adequate lymph node retrieval and complete tumor resection are essential for accurate staging and prognosis in esophageal cancer [19]. The improved visualization and enhanced surgical dexterity provided by the robotic platform may facilitate more thorough lymph node dissection and precise tumor resection. The comparable long-term oncological outcomes, including overall survival and disease-free survival, between robotic and laparoscopic esophagectomy suggest that both approaches can provide similar oncological efficacy when performed by experienced surgeons. This finding is consistent with previous studies that have reported similar long-term outcomes between the two minimally invasive techniques [20, 21]. The strengths of this meta-analysis include the comprehensive literature search, the inclusion of both RCTs and observational studies, and the assessment of publication bias. The rigorous risk of bias assessment and the use of random-effects models to account for potential heterogeneity further enhance the reliability of the findings. However, this study is not without limitations. First, the included studies were conducted in different geographic regions, which may introduce variations in surgical techniques, patient populations, and perioperative management. Second, the sample sizes of the individual studies were relatively small, which may have limited the statistical power to detect small differences between the two approaches. Third, the substantial heterogeneity observed for some outcomes, such as operative time and estimated blood loss, suggests that underlying differences in surgeon experience, patient characteristics, or other unmeasured factors might have influenced the results. Additionally, it should be noted that R0 resection is often determined more by tumor stage and case complexity than by the surgical technique itself, which was not fully explored in this analysis. Finally, the sources of the cited data (e.g., specific databases) were not consistently reported across studies, which may hinder reproducibility and further literature retrieval.
Conclusions
This meta-analysis demonstrates that robotic esophagectomy, despite longer operative times, offers advantages in terms of reduced blood loss, higher lymph node yield, and improved R0 resection rates compared to laparoscopic esophagectomy. Both techniques exhibit comparable postoperative complication rates, length of hospital stay, and long-term oncological outcomes. The choice between laparoscopic and robotic esophagectomy should be guided by surgeon expertise, patient-specific factors, and institutional resources. Future studies should focus on long-term oncological outcomes and cost-effectiveness analyses to further inform clinical decision-making.






