Introduction
Urethral stricture is a partial or complete narrowing of the urethra. It can be caused by trauma or inflammation, which leads to irreversible development of urinary incontinence and, possibly, death. The treatment still remains a challenge [1, 2].
The bulbo-membranous part of the urethra (BMU) is especially susceptible to iatrogenic trauma. Risk factors are the anatomical and functional features of the BMU: the right angle of bend, the smallest diameter in comparison with other parts of the urethra, involuntarily or voluntarily (in patients’ consciousness) closed external urinary sphincter [3, 4].
Diagnosis of urethral stricture is based on subjective complaints, anamnesis, objective examination, and additional and instrumental examination methods. The main specific diagnostic procedures are uroflowmetry, urethroscopy, and urethrography. Additionally, urethral sonography, computed tomography (CT) urethrography or magnetic resonance (MR) urethrography can be performed. The symptoms of urinary incontinence in combination with uroflowmetry and ultrasound examination results increase the possibility of identifying patients with urethral stricture who need to undergo urethrocystoscopy or urethrography to confirm the diagnosis [1, 2, 5, 6].
Currently, there are two methods of surgical treatment of urethral stricture: endourological surgery and urethroplastic surgery [1, 7].
Most often, when a stricture is diagnosed, endourological surgery – direct vision internal urethrotomy (DVIU) – is performed, with approximate effectiveness varying from 10% to 75% [8, 9]. This method is simple and the most effective for strictures of less than 10 mm in length. For strictures of more than 2 cm in length, DVIU is ineffective [10]. The initial and repeated use of this technique can even worsen the course of the disease [9]. More than 90% of such patients in long-term observation suffered from a disease relapse within the next 5 years [11].
An alternative to internal urethrotomy is one of the methods of anastomotic urethroplasty. Usually, a bulbo-membranous anastomosis is performed (on condition of an intact membranous urethra), which is accompanied by the intersection of the spongy body, the contravention vascularization, and innervation [12]. In case of a longer lesion and involvement of the membranous part of the urethra, the most common and effective method is the bulbo-prostatic anastomosis (86% of successful operations) [13]. The method involves excision of the affected part of the urethra, which leads to its shortening. Webster’s techniques aimed at tension-free anastomosis increase the risk of vascular and nerve injury [14]. This type of operation can lead to erectile dysfunction, urinary incontinence and shortening of the penis [13]. Nevertheless, there are controversial data confirming the risk of developing erectile dysfunction as well as in some cases its improvement or recovery over time [13, 15–18].
There are several options for vascular-preserving anastomotic procedures that allow one to avoid dysfunction of the normal blood supply to the spongy body and successfully perform urethroplastic surgery of distal urethral strictures [19, 20]. Due to the technical features these methods are limitedly applicable for strictures of more than 2–3 cm of length. The Jordan operation can be performed to a limited extent for the bulbo-membranous part of the urethra [20]. This technique allows abduction of the vascular bundle and urethral bulb without their intersection. After excision of the urethral bulb, along with the affected area of the urethra, between the distal end and the membrane an anastomosis is applied. The first publications reported more effective preservation of erectile function compared to traditional anastomotic surgery; however, analysis of a number of studies showed that with similar effectiveness there is a continuing risk of erectile dysfunction development [21]. Thus, there is a need for rigorous evidence-based research to present the results of applying such operations more accurately.
Urethroplasty of bulbo-membranous urethral strictures can be used beside anastomotic urethroplasty. It is the replacement of part of the urethra with autologous, allogeneic, xenogeneic or tissue-engineering material. This method is one of the most effective for urethral plastics, but with BMUS its effectiveness concedes to anastomotic methods [7, 13].
Aim
The purpose of the study is a comparative analysis of the anastomotic urethroplasty operations with or without vascular preservation for the BMUs.
Material and methods
Ethical agreement
The clinical research was approved by the local ethics committees of Irkutsk State Clinical Hospital and the Regional State Autonomous Healthcare Institution Irkutsk State Medical University. A prospective, blind, randomized, single-center study was performed in the urological department of Irkutsk State Clinical Hospital.
The clinical part of the research includes an analysis of the examination and treatment results of patients who underwent surgical procedures for strictures (narrowing) of the bulbo-membranous part of the urethra from April 2012 to February 2018.
Patients
All the participants were male, aged older than 18 years with a diagnosis of stricture of the bulbo-membranous urethra. The division of patients in the comparison groups was random before treatment, and the results of randomization were hidden from the patients. Recruitment of patients in the study fitting the inclusion criteria was carried out prospectively by the sampling method until the desired sample quantity was reached.
Over the specified period of time, 272 patients were diagnosed with urethral stricture. Fifty-one patients fit the criteria for inclusion in the research. All included recipients were divided into two groups based on the approved study protocol. If relapse occurred, the patient was asked to re-participate in the study.
Of the 51 patients first included in both comparison groups, 23 were excluded from the research. In the group of excluded patients, 13 dropped out due to deviations from the research protocol, and 10 due to personal reasons.
The criteria for inclusion in the study were:
the patient is scheduled for urethral stricture plastic surgery;
urethral stricture is located in the proximal part of the bulbar and/or membranous parts of the urethra;
patients older than 18 years;
the patient has signed a voluntary consent form to participate in the research;
the patient is to undergo a random method of urethroplasty before the day of surgery.
Criteria for not inclusion:
strictures of the anterior urethra;
the patient has not signed a voluntary consent form to participate in the research;
prostatic hyperplasia, significantly affecting urodynamics;
Exclusion criteria:
the patient refused to participate at any stage of the research;
due to any reason the patient has not undergone surgery or has undergone another procedure that does not meet the criteria of the group.
The endpoints of the study were established.
The primary “solid” endpoints were: the absence of urethral stricture relapse in the long-term postoperative period, but not earlier than 3 months later; relapse detected at any stage of postoperative observation.
Secondary “soft” endpoints of clinical effectiveness were the postoperative examination data: maximum urine flow rate of more than 12 ml/s, residual urine volume of less than 50 ml, indicators of the IIEF-5, IPSS, QoL scales, no signs of relapse based on urethrography results (urethral lumen diameter more than 4 mm).
Diagnostic methods
Anamnestic, clinical, biochemical, radiological, ultrasound, magnetic resonance, and endoscopic methods of research were used during the examination.
Anastomotic urethroplasty was performed using one of two methods: full mobilization of the spongy body bulb (excision and primary anastomosis urethroplasty (Turner-Warwick) – EPA-TWW) and a vascular-preserving method when the spongy body does not intersect (Jordan operation – EPA-J). The anastomotic urethroplasty surgery method was selected randomly for each patient.
During EPA-TWW and EPA-J the patients were in a modified lithotomy position. To perform the operation Y-shaped perineal access was used.
For tension-free anastomosis (EPA), the bulbar part of the urethra by blunt and acute ways was separated from the surrounding tissues in the proximal (to the membranous part of the urethra) and distal (to the suspensory ligament of the penis) directions. The process included several manipulations: the bulbospongiosus muscle separation from the transverse muscles of the perineum, attached to the tendon center of the perineum. For the pelvic diaphragm manipulations, the named muscles were withdrawn posteriorly and to the sides until the penis roots were exposed. After that there was the bulbar artery crossing. It is better to avoid the piercing of these vessels because of their proximity to the nerves in the cavernous bodies’ area.
The urethra crossing was distal to the narrowing and isolated to the penis suspensory ligament (but no further). It is necessary to be careful with the spongy tissue at this point since only the distal source of blood supply remains.
There was a longitudinal section of the membranous urethra dorsal wall to the seminal mound and the ventral wall of the distal urethra. The catheter or urethrocystoscopy helped to check the urethral patency. The ends of the urethra were sewn together with a synthetic absorbable material (vicryl or monocryl 4-6/0), matching the edges of the mucous membrane. The needle moved from the outside to the inside. A needle holder helped to capture the end of the needle and to stretch the thread through the prostatic wall of the urethra. The first suture was applied along the dorsal wall in the 12 o’clock direction (the standard hour dial in the patient’s back position), and then another 6–10 stitches, moving clockwise. The threads followed the same order as the stitches. It is important to match the edges of the mucous membrane. To reduce the tension, there were several sutures on the adventitia of the urethra ends. The bulbospongiosus muscle filled the remaining pockets.
Webster techniques helped to form new space for the urethra in case of anastomosis tension (the bulbar urethra extended mobilization, the interstitial septum dissection, rerouting – moving the urethra over the cavernous body of the penis roots, lower pubectomy).
If after the bulbar urethra extended mobilization in the distal direction the risk of anastomosis tension remained, the cavernous bodies were separated by 4–5 cm distal to the penis root. This division did not deviate from the median line of the perineum since there was a risk of injury to the neurovascular bundles located on the dorsal surface of the cavernous bodies.
Webster’s next technique is lower pubectomy. Soft tissues were detached from one of the cavernous bodies, trying not to damage the adjacent neurovascular bundle. To form a recess corresponding to the outer diameter of the urethra in the pubic bone the periosteum of the pubic bone was cut along the median line, and it was removed with a dissector, trying not to damage the vessels. Subsequently, an osteotome and bone cutters helped to perform a wedge-shaped resection of the lower branch of the pubic bone and the urethral edges anastomosis.
Rerouting is another possible Webster technique aimed at the deficit correction of the urethra length. The urethra skirted one of the cavernous bodies. This manipulation can cause a small rotation of the penis around the axis.
There are several vascular-preserving anastomotic procedures. This study incorporates the Jordan operation, as it is the best method for the affected area excision and anastomosis of the urethra resulting ends.
During an anastomosis without crossing the spongy body (operation according to Jordan G.H.), the access was in a similar way to Webster’s operation. The difference was that at the urethral mobilization stage, the proximal part of the bulb was isolated to the level of the vessels without crossing them. Then the bulbar urethra was mobilized along the dorsal surface with a bulbar body reversal.
The urethra was dissected along the dorsal surface in the BMU part above the stricture. The spongy body was removed on vascular holders along with the previously isolated vessels. The altered scar tissue in the urethra was completely excised.
The further course of the operation was similar: a standard anastomosis of the spatulated edges of the urethra and a layer-by-layer suture.
Jordan’s surgical technique is not recommended for strictures longer than 2–3 cm, since re-routing is impossible to perform, and other Webster techniques are difficult.
An anamnestic study was performed in order to establish the possible cause and duration of the disease.
Laboratory tests included clinical analysis of blood and urine, determination of total protein, blood sugar, creatinine, urea, bilirubin, amylase and transaminase activity, and water-electrolyte balance in the blood. All patients underwent a bacteriological urine test, electrocardiography, and ultrasound of the urinary tract with an assessment of the volume of residual urine.
Erectile function (EF) was evaluated according to the IIEF-5 scale for subsequent comparison of the influence of urethroplasty methods on it. To increase the accuracy of the analysis of erectile function, the factor of taking medications that may suppress it was also considered (antiandrogenic, sedative and vasoactive). Quality of life (QoL) was evaluated by patients subjectively (from 0 points – excellent; to 6 points – very poor). According to the IPSS scale severity of symptoms from the lower urinary tract distinguishes three categories: 7 or less – mildly symptomatic, 8–19 – moderately symptomatic, 20–35 – severely symptomatic. The severity of postoperative pain was evaluated according to the Visual Analogue Scale. The assessment of these subjective symptoms was carried out by patients on their own using standardized questionnaire forms.
To clarify the nature and extent of pathological changes in the urethra all patients underwent uroflowmetry, urethrography, urethrocystoscopy, or urethral calibration. In doubtful cases, additional MSCT or MR urethrography was performed with 3D image reconstruction for the final verification of the diagnosis.
Uroflowmetry was performed according to a standard procedure with a recommended bladder filling volume of 250–500 ml. Ultrasound examination was performed in standard positions. The volume of residual urine was evaluated in two stages: determining the volume of the bladder before and after urination. Urethroscopy was performed in an operating room or cystoscopy room.
Urethrocystography was performed in an X-ray room or MR and MSCT rooms. The research was performed according to standard methods (retrograde, cystographic, and mictational phases; antegrade and retrograde combined urethrocystography). The iodine-containing drug Urografin or Omnipack 300–350 in a volume of 40–60 ml with a dilution of up to 200 ml was used as a contrast substance. During MR urethrography a saline solution of 0.9% NaCl was injected into the urethra.
The 3-month period after the operation all the patients at least once every 6 months underwent the standard medical tests established by the protocol of the research: urologist consultation, clinical blood, and urine tests, urethrography, urethroscopy, uroflowmetry, and ultrasound. Subjective status was also evaluated using the IPSS, QoL, and IIEF-5 scales. Patient complaints were recorded.
The effectiveness of the treatment was evaluated according to several criteria: the maximum urine flow rate (by the method of uroflowmetry), the diameter of the urethral lumen in the plastic zone (according to urethrography), the volume of residual urine (ultrasound), and rating scale indicators (IPSS, QoL and IIEF-5). After 3 months or more after surgery the results of treatment of patients with the following parameters were considered successful: Qmax more than 12 ml/s; absence of residual urine or signs of relapse according to urethrography (normal diameter of the urethral lumen in the plastic zone is 5 mm or more); absence of severe symptoms of the lower urinary tract and satisfactory quality of life.
The treatment results were evaluated based on a comprehensive analysis of all primary and secondary endpoints parameters. Absence of relapse and adequate urination do not give an accurate image of the quality of life of the patient after surgery. Therefore, the interpretation of the results requires consideration of all factors.
The treatment results were divided into three groups.
Good result. In this group no relapse was recorded during the entire observation period, the maximum urine flow rate more than 12 ml/s, residual urine volume of less than 50 ml, no signs of relapse according to urethrocystography and urethroscopy (diameter of the urethral lumen in the plastic zone is more than 4 mm), as well as with retained urine retention, preserved or slightly reduced erectile function (15 or more points on the IIEF-5 scale), no complaints of penis shortening after surgery, absence of symptoms (a score of 19 or less on the IPSS scale), and a satisfactory quality of life (more than 3 on the QoL scale) on subjective assessment.
A satisfactory result was considered for the absence of relapse during the observation period, the maximum urine flow rate more than 12 ml/s, residual urine volume less than 50 ml, no signs of relapse according to urethrocystography and urethroscopy (urethral lumen diameter in the plastic zone is more than 4 mm). Also, this group included patients with any of the following negative consequences: incontinence or moderate erectile dysfunction (less than 15 points on the IIEF-5 scale), complaints on shortening of the penis after plastic surgery, an unsatisfactory subjective assessment of quality of life (4 or less points on the QoL scale) and severely symptomatic (20 or more points on the IPSS scale).
An unsatisfactory result was considered for the absence of adequate urination (less than 12 ml/s), significant residual urine volume (more than 50 ml), clear signs of relapse according to urethrocystography and urethroscopy (urethral lumen diameter in the plastic zone less than 4 mm), regardless of subjective assessment condition, continent and erectile function.
Statistical analysis
The initial data and the results of surgical treatment were analyzed using Statistica software for Windows version 10.0 (StatSoft, Inc, USA), SPSS Statistics version 23.0 (IBM, USA) and Stata version 14.2 (StataCorp, USA).
Continuous variables were expressed as mean ± SD or median and interquartile ranges. Categorical variables were expressed as frequencies and percentages. Comparison of parametric continuous variables was performed using Student’s t test. The Mann-Whitney U test was used for comparison of non-parametric variables. The χ2 test was used in the comparison of categorical variables. P-values of 0.05 or less were considered as statistically significant.
Results
Preoperative parameters
EPA-TWW group
A classic anastomosis (Turner-Warwick operation) with complete mobilization and cutting off the spongy body bulb was performed for 15 patients (EPA-TWW; n = 15; 53.5%). The age of the patients was significantly different. There was 1 patient younger than 20 years, 3 patients aged from 20 to 40 years, 6 patients aged from 40 to 60 years and 5 patients older than 60 years. The average age of the patients was 50.5 ±17.9 years (95% CI: 13.1–28.3), the minimum was 18 years, and the maximum was 76 years. The duration of the disease was also analyzed. For 7 patients the anamnestic period was up to 1 year, for one – up to 2 years, for one – up to 5 years, for one – up to 10 years and for 5 patients – more than 10 years. The average disease duration was 1.5 (0.6; 21) years (95% CI: 8.2–17.7). The shortest period was one month, the maximum 32 years.
For some patients, concomitant pathology affecting the function of the genitourinary system was diagnosed. Sometimes it was combined. Four (26.6%) patients were diagnosed with benign prostatic hyperplasia (BHP), 7 (46.6%) with urethritis, 4 (26.6%) with ischemic heart disease (IHD), 6 (40%) with pelvic venous congestion, 1 (6.6%) with preoperative incontinence.
An anamnestic analysis revealed that iatrogenic (n = 7; 46.6%) and traumatic (n = 12; 80%) genesis could equally cause the formation of urethral strictures (p = 0.366).
Previously, 11 (73.3%) patients underwent procedures involving the urethra. Three (20%) patients underwent transurethral interventions (DVIU, repeated catheterization, TURP of the pancreas and bladder, ureteroscopy), 3 (20%) – operations on the pancreas, 8 (53.3%) – urethral plastic surgery.
Residual urine volume was significant (over 100 ml) for 7 patients, moderate (less than 100 ml) for 4 patients, and for 3 patients there was no residual urine. Residual urine volume ranged from 0 ml to 400 ml. The average value was 180 (40; 223) ml (95% CI: 90–194).
According to the uroflowmetry data, for 5 patients the urine flow rate was critically low (less than 3 ml/s), for 7 there was pronounced reduction (from 3 to 5 ml/s), and it was moderately reduced (from 5 to 10 ml/s) for 3 patients. The minimum value of Qmax was urination dropwise (0–1 ml/s), the maximum – 6 ml/s.
The extent of urethral strictures was evaluated by urethrography and urethroscopy. The results showed short strictures (less than 10 mm in length) for 4 patients, medium-length (from 11 to 20 mm) for 6, and long strictures (over 21 mm) for 5 patients. The minimum length of the stricture was 4 mm, the maximum 50 mm.
Complete obliteration of the urethral lumen was diagnosed for 4 patients, subtotal narrowing (0–1 mm) for 7, pronounced narrowing (1–4 mm) for 4 patients. The average diameter of the urethral lumen in the stricture location was 1 (0; 2) mm (95% CI: 0.9–2.1).
EPA-J group
A vessel-sparing method without crossing the spongy body (Jordan operation) was performed for 13 patients (EPA-J; n = 13; 46.4%) (p = 0.757). The EPA-J group included 13 patients. The age of the patients was significantly different. From 20 to 40 years – 1 patient; from 40 to 60 years old – 5; and older than 60 years – 7 patients. The average age of the patients was 61.3 ±15.2 years (95% CI: 10.9–25.1), the minimum was 25 years, and the maximum was 77 years.
An analysis of the disease duration was also performed. For 4 patients the anamnestic period was up to one year, for 3 it was up to 2 years, for 4 it was up to 5 years, and for 2 patients it was more than 10 years. The average disease duration was 2 (1; 4) years (95% CI: 7.2–16.6). The shortest period was 1 day, the longest 37 years.
Drugs causing the side effect leading to erectile dysfunction were taken by 7 (53.8%) patients, α-adrenergic blockers by 7 (53.8%) patients.
Some patients had a concomitant pathology that affected the function of the genitourinary system. BPH was diagnosed in 8 (61.5%) patients, urethritis in 9 (69%), IHD in 7 (53.8%), pelvic venous congestion in 5 (38.4%); preoperative incontinence was not found in any of the patients.
An anamnestic analysis revealed that iatrogenic (n = 9; 69.2%) and traumatic (n = 6; 46.1%) genesis could equally cause the urethral strictures (p = 0.536).
Previously, 10 (76.9%) patients underwent operations involving the urethra. Transurethral interventions (DVIU, multiple catheterization, TURP of the pancreas and bladder, ureteroscopy) were performed in 4 (30.7%) patients, other operations on the pancreas in 5 (38.4%), urethroplasty in 1 (7.6%) patients.
Residual urine volume was significant (over 100 ml) in 10 patients, moderate (less than 100 ml) in one, and in 2 patients there was no residual urine. Residual urine volume ranged from 0 ml to 700 ml.
According to the uroflowmetry data, the urine flow rate was critically low (less than 3 ml/s) in 5 patients, considerably reduced (from 3 to 5 ml/s) in 6, moderately reduced (from 5 to 10 ml/s) in one and slightly reduced (from 10 to 12 ml/s) in 1 patient. The minimum value of Qmax was urination dropwise (0–1 ml/s), the maximum was 11 ml/s.
The extent of urethral strictures was evaluated according to urethrography and urethroscopy. The results showed a short extent in 4 patients (less than 10 mm in length), medium-length (from 11 to 20 mm) in 5 patients and extended (more than 21 mm) strictures were found in 4 patients. The minimum length of the stricture was 3 mm, the maximum 30 mm. The average length of the strictures was 18.5 ±8.9 mm (95% CI: 6.3–14.7).
Complete obliteration of the urethral lumen was revealed in 3 patients, subtotal (0–1 mm) narrowing in 6 patients, pronounced (1–4 mm) narrowing in 4 patients. The average diameter of the urethral lumen in the stricture location was 1 (1; 2) mm (95% CI: 0.9–2.2).
A comparative analysis of the preoperative parameters is presented in Table I.
Table I
Comparison of initial clinical parameters of patients after EPA-TWW or EPA-J
Condition of patients undergoing anastomotic surgery using EPA-TWW and EPA-J methods was compared according to the initial clinical parameters (the results are presented in Table I as values for patients undergoing EPA-TWW and EPA-J with an indication of p). According to the results of the analysis, the preoperative characteristics of patients undergoing both methods of anastomosis were comparable.
Postoperative results analysis
EPA-TWW group
Objective and subjective parameters of the effectiveness of the surgical treatment of 15 patients after EPA-TWW were evaluated according to the criteria established in the research.
This method of anastomosis was accepted as effective according to the criterion of absence from disease recurrence no earlier than 3 months after surgery in 80.0% (n = 12) of cases.
For a comprehensive evaluation of anastomotic urethroplasty results using the EPA-TWW method, the data of the endpoints were analyzed: absence of relapse during the observation period (n = 12; 80%), the adequacy of self-urination (n = 12; 80%), and the absence of residual urine after urination (n = 12; 80%), quality of life (2 or more points, n = 11; 73.3%), erectile function (15 or more points, n = 3; 20%), severity of symptoms (19 or less points, n = 13; 86.6%), as well as postoperative incontinence (n = 2, 13.3%) and complaints about shortening of the penis (n = 3; 20%).
Thus, after EPA-TWW good treatment results were obtained for 3 (20%) patients, satisfactory for 9 (60%) cases. Unsatisfactory results were recorded for 3 (20%) patients.
EPA-J group
Objective and subjective postoperative parameters of surgical treatment effectiveness of 13 patients after EPA-J were evaluated according to the criteria established in the research.
This method of anastomosis was accepted as effective according to the criterion of absence from disease recurrence no earlier than 3 months after surgery in 92.3% (n = 12) of cases. Postoperative parameters of patients in this group are shown in Table II.
Table II
Comparison of postoperative parameters of patients after surgical treatment with EPA-TWW and EPA-J methods
For a comprehensive assessment of the anastomotic urethroplasty results using the EPA-J method, the data of the study endpoints were analyzed: absence of relapse during the observation period (n = 12; 92.3%), the adequacy of independent urination (n = 13; 86.6%), and the absence of residual urine after urination (n = 12; 92.3%), quality of life (2 or more points, n = 9; 69.2%), erectile function (15 or more points, n = 3; 23%), the severity of symptoms (19 and less points, n = 12; 92.3%), as well as postoperative incontinence (n = 3, 23%) and complaints about shortening of the penis (n = 1; 7.6%).
Thus, after EPA-J good treatment results were obtained for 3 (23%) patients, satisfactory for 9 (69.2%) cases. Unsatisfactory results were recorded for 1 (7.6%) patient.
Fifty patients underwent a classic anastomosis (Turner-Warwick operation) with complete mobilization and cutting off the spongy body bulb (EPA-TWW; n = 15; 53.5%), and without crossing the spongy body (Jordan operation) – 13 (EPA-J; n = 13; 46.4%) patients (p = 0.757).
Comparison of postoperative parameters in study groups
A comparative analysis of the postoperative parameters of patients after EPA-TWW and EPA-J is shown in Table II.
A comparison was made of patients undergoing anastomotic surgery using EPA-TWW and EPA-J methods according to postoperative parameters (the results are presented in Table II as values for patients undergoing EPA-TWW and EPA-J with an indication of p). According to the results of the analysis, the postoperative parameters of patients who underwent both methods of anastomosis were also comparable.
Figure 1 presents an intergroup statistical analysis of the changes in the functional status and quality of urination in patients undergoing surgical treatment.
Figure 1
Comparison of changes in functional status and quality of urination after plastic surgery using the EPA-TWW (1) and EPA-J (2) methods
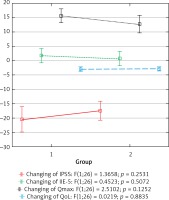
Postoperative changes in the parameters of the functional status of patients based on the IPSS scales (F (1; 26) = 1.3558; p = 0.2531), IIEF-5 (F (1; 26) = 0.4523; p = 0.5072), QoL (F(1; 26) = 0.0219; p = 0.8835), as well as the maximum urine flow rate increase (Qmax: F(1; 26) = 2.5102; p = 0.1252) were comparable for patients undergoing EPA-TWW and EPA-J.
The effectiveness of the classical anastomosis method of EPA-TWW (80%) was comparable (p = 0.797) with the method of anastomosis without crossing the spongy body of EPA-J (92.3%).
Based on a comparative analysis conducted between groups of EPA-TWW and EPA-J patients, their statistical equality was established.
Multivariate logistic regression analysis helped to determine the predictors of postoperative complications. Predictor variables were selected based on initial parameters (more than 200). The tables show a sample and statistically significant results of the analysis. Table III presents the predictor factors of postoperative complications data (multivariate logistic regression analysis).
Table III
Postoperative complications predictors
[i] US – urinary system, STIs – sexually transmitted infections, Qmax – maximum urine flow rate, LUTS – lower urinary tract symptoms, LUT – lower urinary tract, TU –transurethral, ED – erectile dysfunction, CVI – chronic venous insufficiency, AMI – acute myocardial infarction, EPA – anastomotic surgery, BMG – substitutional intraurethral plastic.
Incontinence predictors after anastomotic surgery showed the significance of previous operations on the prostate (OR = 2.4; 95% CI: 0.2–4.9; p = 0.049). A multivariate logit regression showed no statistical correlation.
A simple logistic regression analysis of the penile shortening risk revealed the impact of undergoing anastomotic plastic surgery of more than 2 cm urethral defect length (OR = 1.7; 95% CI: 0.1–3.4; p = 0.037) and long-term catheterization in anamnesis (OR = 0.2; 95% CI: 0.1–0.7; p = 0.031). A multivariate logit regression showed no statistical correlation.
A multivariate logit regression showed no statistical correlation between the IIEF5 and the surgical treatment methods and preoperative parameters. However, in simple analysis, previous pelvic venous fullness can have a possible negative effect (OR = 2.11; 95% CI: 3.9–0.28; p = 0.024).
Discussion
Strictures of the bulbo-membranous urethra are one of the main causes of obstructive urination. Current trends in the development of medicine lead to wider use of endoscopic methods and more frequent iatrogenic injury of the urethra.
The method of anastomotic plastic surgery of the urethra BMU, recommended by the largest world urological associations (European, American, and Russian), is highly effective (from 85% to 98%), but its technical features can lead to undesirable complications. Surgical techniques used in existing plastic surgery lead to significant damage to the anatomical structures of the genitourinary system and perineum during access, mobilization of the urethra, and dissection of the altered tissues. Such surgical intervention allows successful restoration of the urethra, on the one hand, and, on the other hand, leads to persistent, difficult to correct further complications (urinary incontinence, impaired sexual function and, as a result, reduced quality of life).
Other things being equal, such as group homogeneity and a single surgical correction algorithm, restoration of normal urethral patency was achieved in both study groups. Thus, in the absence of recurrence of stricture disease, treatment for patients in both groups was successful.
The final data indicate a statistically equivalent risk of developing complications such as urinary incontinence (2 (13.3%) after EPA-TWW and 2 (15.3%) after EPA-J, p = 0.893), shortening of the penis (3 (20%) after EPA-TWW and 1 (7.6%) after EPA-J, p = 0.419), decreased erectile function (9 (60%) after EPA-TWW and 4 (30.7%) after EPA-J, p = 0.343). However, an informal assessment demonstrates the best state of erectile function for patients after vascular-preserving surgery (60% vs. 30.7% decrease in erectile function).
Both methods showed zero hospital mortality and the absence of serious complications (cardiovascular incidents, cerebrovascular accidents, respiratory failure, hemorrhagic complications).
Urethroplasty operations in the bulbomembranous part is a difficult task [22]. Classical anastomotic urethroplasty for short strictures with blood vessels’ preservation showed comparable results of efficiency from 85% to 98% [23–25].
Anastomotic urethroplasty with blood vessels’ preservation in the proximal part of the bulbar urethra or in the posterior (membranous) urethra showed similar results (93.4% and 88.5%, respectively) with a comparable risk of complications. Thus, from a clinical point of view, combining these anatomical groups into a single part – the bulbomembranous urethra – is appropriate.
The largest studies [11, 15, 26] indicate a significant risk of erectile dysfunction and urinary incontinence after urethroplasty. There was no statistically significant difference in the risk of erectile dysfunction between the methods of urethroplasty itself.
Performing vascular-preserving urethroplasty for strictures of the bulbo-membranous urethral part is also connected with a risk of erectile dysfunction (more than 13.4% according to a meta-analysis of 10 out of 14 large studies), as well as performing traditional anastomotic surgery [14]. There are also conflicting results and evidence of a gradual improvement in erectile function over time [13, 15, 16]. The expected recovery of erectile function after surgical treatment is estimated to take more than 1 year, and is about 80% after 6 months and 90% after 1 year from the initial level of IIEF-5 [11]. According to the study, no reliable results have been obtained indicating a gradual restoration of erectile function. This problem requires further study.
Our findings are consistent with the literature, confirming the negative impact of common surgical techniques for performing urethroplasty for erectile function and urinary retention, and also confirming the feasibility of performing vascular-preserving techniques.
Conclusions
The research did not demonstrate a statistically significant difference in the effectiveness of the treatment and the risks of developing complications when performing anastomotic surgery with or without vascular preservation. However, an informal assessment demonstrates the best state of erectile function after vascular-preserving surgery.









