Intraoperative arterial blood pressure (BP) lability refers to deviations beyond the accepted physio-logical range or to rapid changes in arterial BP that commonly occur during anaesthesia in noncardiac surgery [1, 2]. The presence of BP lability alerts the anaesthetists to potentially deleterious conditions, such as hypovolaemia, inadequate depth of anaesthesia, and the potential for cardiovascular complications. Intraoperative BP lability has been reported to be a prognostic factor for postoperative adverse outcomes [3] and mortality [4] in noncardiac surgery. Also, the consequence of perioperative haemodynamic instability, both intraoperative hypertension and intraoperative hypotension, have been reported to be associated with perioperative vital organ complications, such as myocardial injury, acute kidney injury, postsurgical delirium, intracranial haemorrhage, and death [4–13]. During anaesthesia, BP should be maintained within 20% of the best estimate of the preoperative baseline [9, 12, 14].
Hypertensive patients are known to have increased arterial BP lability during anaesthesia compared with normotensive patients [1]. Because these patients are usually associated with an increased systemic vascular resistance when anaesthesia is induced, systemic vasodilatation will occur, and this will be expected to have profound effects on arterial pressure. On the other hand, hypertensive patients, especially those who are untreated or whose hypertension is inadequately controlled, have a more vigorous cardiovascular response to noxious stimuli [15].
Masked uncontrolled hypertension occurs in approximately 30% of hypertensive patients on antihypertensive therapy in whom BP appears to be adequately controlled based on clinic assessment [16, 17], but whose BP at home remains high. These patients are under-detected at the clinic. The risks of future cardiovascular events and vital organ damage in masked hypertensive patients are, significantly, 2–3 times higher than in normotension or white coat hypertension [18] and are approximately equal to those of patients with sustained hypertension [19]; identification of masked uncontrolled hypertensive patients should be of concern before hospitalization for elective surgery.
Because of the controlled hypertension at a cli-nic, masked uncontrolled hypertensive patients are at high cardiovascular risk but are underdiagnosed in the preoperative period, and their BP response, including BP lability, during anaesthesia and surgery has not been well documented. This study was conducted to compare the magnitude of BP lability during general anaesthesia in treated hypertensive patients who have adequately controlled BP based on clinic measurements between masked uncontrolled hypertension and adequately controlled BP. The magnitude of intraoperative BP lability during general anaesthesia was considered the primary outcome; the magnitude of BP lability during induction and postoperative periods was considered a secondary outcome.
METHODS
In this prospective observational study, a comparison of arterial BP lability during general anaes-thesia was measured and compared between masked uncontrolled hypertensive and adequately controlled hypertensive patients. Approval was granted by the Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Thailand (REC.62-387-18-5), and the proposal was submitted to the Thai Clinical Trial Registry (TCTR2021-0615001).
Patient selection
Patients aged 18 to 85 years with currently treated hypertension, who had apparently controlled BP based on office measurement (less than 140/90 mmHg) and were scheduled for elective major noncardiac surgery under general anaesthesia, were invited to participate between August 2020 and January 2021. All patients gave their written informed consent after receiving essential information about the study objectives. Those with a history of severe cardiac problems, e.g. severe valvular heart disease, heart failure, myocardial infarction with poor cardiac output, left ventricular ejection fraction (LVEF) < 35% or functional class III–IV by New York Heart Association (NYHA), pheochromocytoma or mass at adrenal gland, increased intracranial pressure, end-stage renal failure with haemodialysis or peritoneal dialysis therapy, severe vascular diseases, e.g. aortic aneurysm, arterial occlusion, or pregnancy, were excluded.
Study protocol
At the outpatient clinic, the baseline BP in the clinic was calculated from BP measurements at the last 2 outpatient visits taken with the patient unstressed, pain-free, and in a sitting position. The baseline clinical profile of all participants was detailed, and the patients’ atherosclerotic cardiovascular disease event or risk [20] was assessed using the Thai cardiovascular risk score [21] as a tool to estimate the 10-year risk for atherosclerotic cardiovascular disease.
Home BP monitoring (HBPM) was the method selected to measure out-of-office BP, because HBPM involves multiple measurements of BP at different times in the usual environment of each individual [22], has higher diagnostic performance than clinic BP measurement [16], and is often a more practical approach than ambulatory BP monitoring (ABPM) in clinical practice. Every patient and caregiver performed BP self-monitoring by taking and recording at least 2 measurements on one occasion in the morning and 2 measurements on another occasion in the evening over a period of 3-7 days [19, 22] within 6 months prior to surgery at home or elsewhere outside the clinic setting after being trained to follow the protocol by cardiac nurse specialists. Detailed instructions given to each patient are presented in Supplementary Figure 1.
For accurate measurement and recording of BP, all patients used an automatic BP device (HEM-7121AP OMRON Healthcare, Japan), which is clinically validated according to standards of the Association for the Advancement of Medical Instrumentation Standard (AAMI)/European Society of Hypertension International Protocol (ESH) as an HBPM device. Cuff size and the procedures for the BP collection and the measurement validation followed the recommendation of the 2017 ACC/AHA High BP Clinical Practice Guideline [19].
The investigator checked the accuracy of HBPM records by confirming against the electronic data storage in the device. The BP value on each occasion used for subsequent analysis was selected after first excluding artefacts, recognized as systolic blood pressure (SBP) values < 50 mmHg or > 250 mmHg or diastolic blood pressure (DBP) values < 20 mmHg or > 150 mmHg, and then taking the 2nd measurement if 2 readings were recorded or the mean of the 2nd and 3rd if 3 readings were recorded [19]. Daytime means were then calculated using the selected morning and evening values over all monitoring days.
All patients had a mean clinic BP of < 140/90 mmHg. Masked uncontrolled hypertension patients were distinguished by having a mean daytime BP from multiple measurements using HBPM ≥ 135/85 mmHg [17, 19]. Thus, patients were classified into 2 groups: masked uncontrolled hypertension and adequately controlled hypertension, according to the stated criteria. The classification was confirmed by an experienced cardiologist, who continued to provide care to optimize patients’ BP following the 2017 ACC/AHA guideline for the prevention, detection, evaluation, and management of high BP in adults until the day of surgery.
On the day of operation, the intraoperative BP was monitored either oscillometrically from an upper-arm cuff at intervals or continuously from an arterial catheter, and heart rate was recorded as the actual data from the real-time monitor (IntelliVue MX550/MP50 Patient Monitor, Philips, Germany). Pre-induction vital signs were measured after stabilizing in the operating room for 5–10 minutes before induction of anaesthesia and monitored throughout the procedure. SBP, MAP, and DBP were recorded every minute during the first 10 minutes after the induction and then every 5 minutes until the end of the recovery phase in the Post Anaesthetic Care Unit (PACU).
General anaesthesia was given under the decision-making of the attending anaesthetic team, who did not know the classification of hypertension in that patient. Anaesthesia induction agents used were mostly intravenous propofol 1–3 mg kg–1, fentanyl or morphine as analgesic drugs, and cisatracurium or rocuronium as muscle relaxants for facilitated intubation and immobilization during surgery. All patients were continuously monitored as standard basic anaesthetic monitoring recommended by the American Society of Anaesthesiologists (ASA): ECG, pulse oximetry, heart rate/pulse, arterial BP, capnography, body temperature, and multiple expired gas analysis. During the intra-operative period, depth of anaesthesia was maintained with volatile agents, sevoflurane or desflurane, to keep 1.3–1.6 times the minimal alveolar concentration (MAC) by expired gas analysis monitoring; also, good oxygenation and ventilation were maintained with positive pressure ventilation either volume- or pressure-controlled ventilation setting by keeping the oxygen saturation > 93% and end-tidal carbon dioxide at 30–40 mmHg.
Sample size
The planned sample size required was estimated based on having 80% power to detect a difference in intraoperative BP lability measure between masked uncontrolled and adequately controlled hypertension patients with an effect size of 0.8 as statistically significant at an a of 0.05.
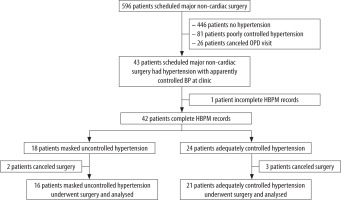
The proportion of masked hypertension among eligible patients was expected to be approximately 35%, thereby requiring a total sample size of 55 patients (19 masked and 36 adequately controlled patients). However, the COVID-19 pandemic intervened, and because of the need for infection control and the limitation of resource allocation, outpatient department (OPD) visits were restricted, and many elective surgeries were cancelled. Finally, a little less than 70% of the planned sample size was available at the time of analysis, meaning that the power was adequate only for a somewhat greater effect size than planned. The participant selection process is depicted in Figure 1.
Data analysis
Most anaesthetists have been educated that arterial BP lability is defined as rapid changes in arterial BP over a short period. However, there is no definitive standard for evaluating BP lability [7]. In this study, the perioperative SBP, MAP, and DBP values were analysed in each of the 3 periods, namely the induction, intraoperative, and immediate postoperative periods in the PACU, and variation in BP was estimated using 3 methods of analysis.
Out-of-range probability relative to patient’s BP baseline [8, 23]. The BP lability was quantified by determining the probability of data points of SBP, MAP, and DBP measurements falling outside the thresholds of –20% to +20% of the patient’s baseline BP. Three different baselines of SBP, MAP, and DBP were used: the patient’s mean at home, in the clinic, and preinduction. The probability of having out-of-range measurements within individual patients was compared between the masked uncontrolled and the adequately controlled groups in each perioperative phase using mixed-effects random-intercept logistic regression.
Standard deviation (SD) [7, 24] and variance (VAR) [25]. The SD and VAR of all BP readings of SBP, MAP, and DBP during the induction, intraoperative, and recovery periods were calculated as a measure of intrapersonal BP lability. Because the distribution of VAR was right-skewed, it was transformed by natural logarithm before analysis. The within-group means of intra-individual SD and intra-individual ln(VAR) were compared between masked uncontrolled hypertensive and adequately controlled hypertensive groups in each perioperative phase using linear regression.
Absolute change from one point to the next [1, 7]. The mean of absolute changes in BP (either decrease or increase) from one point to the next point of measurement was defined as the absolute real variability (ARV) in mmHg. In addition, the sum of absolute BP differences divided by the overall duration of BP readings was used to measure the average absolute variation per unit of time (time-averaged absolute real variability; TARV) in mmHg/minute. The within-group means of individual values of ARV and TARV were compared between groups in each perioperative phase using linear regression.
All data were processed using Stata version 14.1 (StataCorp LLC, Texas, USA). Standard descriptive analysis was performed. Patient characteristics are reported as mean and SD for normally distributed data and were compared using a t-test. Categorical variables are presented as the frequency with percentage and were compared across groups using the chi-square test or Fisher’s exact test. A P-value < 0.05 was considered to indicate statistical significance. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was applied for appropriate data reporting.
RESULTS
Forty-three consecutive eligible patients were enrolled; 37 patients underwent surgery as planned and were analysed in this study. We excluded one patient who could not complete HBPM and 5 patients who cancelled the operation (owing to a changed treatment plan in 3 patients and postponed surgery in 2 patients). An experienced cardiologist categorized all patients to yield a masked group (n = 16) and an adequate group (n = 21).
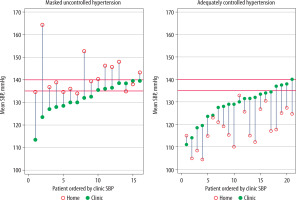
Baseline BP measurements in each group are shown in Table 1 and Figure 2. The group mean and SD of SBP, MAP, and DBP and the difference between home and clinic BP are compared in Table 1. In the masked group, the group mean home SBP and MAP were greater than the corresponding group means in the clinic, in contrast to the adequate group, among whom the group mean home SBP and MAP were lower than the corresponding group means in the clinic. In individual patients, the home SBP was greater than the clinic SBP in the masked group in all but 2 patients whose clinic SBP mean was slightly higher than their home SBP mean but who fulfilled the criteria for being classified as masked uncontrolled hypertension. In addition, among the adequately controlled group, 2 patients exhibited slightly higher home SBP than clinic SBP (Figure 2).
TABLE 1
Mean (SD) baseline blood pressure and the difference in values between home and clinic by type of hypertension
FIGURE 2
Clinic and home baseline systolic blood pressure of individual patients in masked uncontrolled and adequately controlled hypertension groups. Within each group, patients have been ordered according to increasing baseline clinic systolic blood pressure (SBP) values
Baseline characteristics and perioperative variables in masked and adequately controlled groups are compared in Supplementary Table 1. Most variables showed no significant difference between groups; however, the adequate group showed higher current use of angiotensin-converting enzyme inhibitors (ACEIs) as the antihypertensive medication and longer durations of anaesthesia and surgery than the masked group.
Supplementary Figure 2 presents each patient’s SBP, MAP, and DBP throughout the perioperative period and displays the mean individual home BP. The scale of the X-axis in the induction period (first 10 min) has been expanded 5× to show more clearly the fluctuation in BP during that period.
Estimations of BP lability using each of the 3 methods are summarized in Tables 2–4. When using the out-of-range method, no statistically significant difference in BP lability between groups was apparent, whether the home, clinic, or pre-induction baseline was used.
TABLE 2
Estimates of lability of systolic blood pressure by type of hypertension
TABLE 3
Estimates of lability of mean arterial pressure by type of hypertension
TABLE 4
Estimates of lability of diastolic blood pressure by type of hypertension
Using SD or ln(VAR) as a measure of lability indicated higher SBP lability during the intraoperative and postoperative periods in the masked group than in the adequate group (intraoperative period; SD 17.97 [15.33, 20.60] vs. 13.53 [11.22, 15.82], P = 0.014 and postoperative period; SD 10.40 [7.65, 13.16] vs. 5.49 [2.96, 8.02], P = 0.012). Similarly, the SD of intraoperative and postoperative MAP showed significantly higher BP lability (intraoperative period; SD 12.35 [10.70, 13.99] vs. 9.66 [8.22, 11.10], P = 0.017 and postoperative period; SD 7.21 [5.05, 9,38] vs. 4.06 [2.09, 6.05], P = 0.037).
The mean of absolute changes from one point to the next of SBP in the intraoperative period exhibited statistically significant higher values in the masked group than in the adequate group, both with and without averaging over time (12.40 [10.43, 14.37] vs. 9.50 [7.78, 11.22], P = 0.031 in ARV and 2.35 [1.95, 2,74] vs. 1.82 [1.49, 2.16], P = 0.047 in TARV) (Table 2).
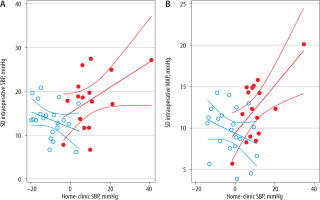
The relationship between intraoperative SD and home-clinic difference in SBP and MAP is demonstrated in Figure 3. Overall, SD appeared to increase with increasing difference between home-minus-clinic difference in the masked group (SBP slope 0.290 [–0.022, 0.602], P = 0.066; MAP slope 0.290 [0.086, 0.490], P = 0.066) but with questionable significance owing to one patient with an extreme home-clinic discrepancy. There was no evidence of an inverse relationship between SD and home vs. clinic discrepancy among adequately controlled hypertensive patients.
FIGURE 3
Scatter plot of intraoperative standard deviation (SD) of blood pressure (BP) against clinic-minus-home baseline BP value. A) SD of systolic blood pressure (SBP) against clinic-minus-home baseline SBP value. B) SD of mean arterial pressure (MAP) against clinic-minus-home baseline MAP value. Solid circles represent masked uncontrolled hypertensive patients, and hollow circles represent adequately controlled hypertensive patients. Patient study numbers within each group correspond to those in Figure 2 and Supplementary Figure 2
One patient in the masked group developed an abnormal ECG with ST elevation and hypotension during the intraoperative period. This was a critical cardiovascular event that was a life-threatening condition and needed immediate management to save the patient’s life. Fortunately, the patient survived.
DISCUSSION
Our study found a significantly higher SBP and MAP lability in the masked hypertensive patients during the intraoperative and immediate postoperative periods as measured by SD and ln (VAR), and higher SBP lability during the intraoperative period as measured by ARV and TARV, compared to the corresponding values in adequately controlled hypertensive patients. This increased lability implies that not only are masked uncontrolled hypertensive patients at increased risk of future cardiovascular events and vital organ damage [16] but also at risk of difficult intraoperative BP management and perioperative cardiovascular complications. This is consistent with the previously reported findings of increased risk of postoperative complications associated with intraoperative BP variability [4, 12].
Haemodynamic response during the induction of anaesthesia comprises a progressive decline in BP after administration of the induction agents, especially intravenous propofol. Subsequent airway manipulation and intubation can cause the BP to rise and then drop again until surgical incision or noxious stimuli occur. During the intraoperative period, BP may decline due to many factors, including the effects of volatile anaesthetic agents, inhibition of the sympathetic nervous system, and loss of the baroreceptor reflex control of arterial pressure in hypertensive patients. On the other hand, surgical manipulation, excessive fluid administration, and inadequate depth of anaesthesia can cause a rise in BP. These responses may be more pronounced in patients with poor preoperative control of their hypertension [13] and may be a partial explanation of the higher degree of intraoperative BP lability seen in our masked uncontrolled hypertensive patients.
Although this observational study aimed to explore the magnitude of BP lability throughout the perioperative period, from induction until the end of postoperative care in the PACU, the BP would have been monitored and managed by the attending anaesthesiologist to avoid extremely high or low levels because it was not acceptable to ignore the critical value of BP. Such management would have attenuated the magnitude of lability and particularly that estimated using the method of an out-of-range proportion of measured data points. This may partly explain the failure of the out-of-range method to reveal statistically significant lability even when SD, ln(VAR), ARV, and/or TARV indicated increased BP lability.
In practice, out-of-range criteria for any perioperative BP optimization goals should be tailored to the individual patient, especially in hypertensive patients who have impairment of cerebral autoregulation to maintain stable blood flow despite changes in BP when compared with normotensive patients [27]. Hence, neither fixed absolute BP thresholds nor fixed percentage deviations from baseline are likely to fit all hypertensive patients, and there is no recognized standard for measurement of BP lability. In our study, we used a percentage of BP readings lying outside the range of ± 20% of baseline level of SBP, MAP, and DBP, respectively, to provide an estimate of the magnitude of BP lability. However, using a fixed percentage deviation or absolute BP deviation from baseline means that the level of lability is sensitive to the baseline used, which, in the case of masked hypertensive patients, raises the question of which is an appropriate baseline. To make a fair comparison with adequately controlled hypertensive patients, it would seem that the mean clinic BP would be most suitable in this research context aimed at comparing masked uncontrolled with adequately controlled patients, both of whom appear to be controlled in the clinic setting. Nevertheless, there was little discrepancy between the values obtained using home and clinic baselines, but the use of pre-induction baseline yielded strikingly different estimates of SBP and MAP lability estimates. Although we used all 3 baseline references in the out-of-range method to measure individual periope-rative BP lability, the home BP is considered to be the more usual setting value and more predictive of target organ damage [16, 27].
Unlike the estimation of lability using the out-of-range approach, using SD, and therefore also ln(VAR), is independent of any baseline value, so it is commonly used to measure consecutive changes in the BP of each patient without timing considerations [7]. However, 2 subjects with significantly different BP patterns could have similar SD values.
Estimation using the mean of absolute change from one point of BP measurement to the next was calculated as the sum of absolute differences divided by the number of readings minus one [7] with and without considering time averaging. Average real variability (ARV) was reported to be a reliable variability index for prognostic studies, and to predict cardiovascular events better than the SD index in a population-based study [28]. The SD and absolute point-to-point change in BP methods revealed significant differences in SBP lability between the 2 groups in our study.
The BP profiles were analysed separately for each phase of the surgical procedure. Lability during induction is expected in all patients because anaesthesia induction causes an initial drop of BP as anaes-thetic agents induce systemic vasodilatation, but this is followed by a rise in BP during the airway manipulation and subsequently by a further downward BP trend while waiting for surgical incision [10, 27]. These procedure-related fluctuations during induction may partly explain the failure to identify any significantly increased lability in the masked group during the induction phase compared with the adequately controlled group of patients with any of the methods used. While the numerical values of SD and ln(VAR) of SBP and MAP during BP during induction were higher in the masked group, the differences were not statistically significant. It is noted that there were also fewer data points in the induction phase than in the intraoperative and postoperative phases, during which increased levels of lability in SBP and MAP were seen using the SD method.
Significantly higher intraoperative SBP lability in the masked hypertension group during general anaesthesia was also revealed using the mean of absolute change from one point to the next, both disregarding the time and after averaging over time. This parameter may be of more direct relevance to the manner in which BP is monitored during anaesthesia. A serious change of electrocardiogram with haemodynamic instability occurred in one anaesthetized patient in the masked group during surgery. That event alerted us to the need to identify masked uncontrolled hypertension and undertake more meticulous monitoring with heightened attentiveness when anaesthesia is given to masked hypertensive patients.
While the majority of patients classified as having masked uncontrolled hypertension had a positive home-minus-clinic SBP reading, this value ranged from –3.64 to 40.9, even though all conformed to the criteria used in this study to define masked uncontrolled hypertension. This wide range of home-clinic discrepancies was the motivation for examining the quantitative relationship between intraoperative BP lability and the magnitude of these discrepancies. The possible existence of a positive relationship between intraoperative SD and home-minus-clinic SBP and MAP values needs further investigation in studies with a larger sample size. If such a relationship is confirmed, it suggests that the discrepancy between home and clinic SBP among masked hypertensive patients may be linked to more remarkable intraoperative BP lability in part by a common underlying mechanism, possibly involving variable response to stress or instability of BP feedback control.
Numerous reports have attempted to explain the physiology of perioperative BP, BP measurements, definitions of hypertension and hypotension, and the implications of ambulatory BP on long-term cardiovascular outcomes [10, 27]. However, the perfusion of vital organs and the implications of high and low BP signals in the perioperative period remain poorly characterized. Recent studies have suggested that the complexity of the BP pattern during anaesthesia may be more closely related to the occurrence of major adverse events [29] than variability measured by standard deviation or variance alone [3, 29]. It has also been suggested that the complexity of a physiological system reflects a physiological reserve of adaptability to stress [3], and measurements of complexity, or conversely measures of multiscale entropy [29], may hold more promise for relating BP patterns to the risk of major perioperative adverse events.
Even though the relationship between perioperative BP rapid changes and postoperative complications is complex and controversial, the perioperative strategies to protect patients from adverse events in masked uncontrolled hypertensive patients are still of particular concern. Accurate criteria for the diagnosis of masked hypertension should be identified, optimizing preoperative conditions prior to elective surgery. Meticulous haemodynamic monitoring, cautious drug administration, and active control of haemodynamic fluctuations for adequate perfusion and diminished major adverse end-organ events throughout the perioperative period in masked uncontrolled hypertensive patients are strongly recommended.
A strength of this study is the use of several methods to assess BP lability. However, some limitations should be noted. First, the sample size was small, thereby limiting the power to detect small but clinically relevant differences in lability when using some of the methods. Second, out-of-range measurements were made with only the criterion of ±20% of baseline values, and, as noted above, extreme deviations are likely to be controlled promptly by the attending anaesthesiologist, thereby limiting the sensitivity of the out-of-range method of lability assessment. Third, some patients having home BP values reported to be within the normal range and included in the group of adequately controlled hypertension may have had higher home values if they had been monitored using ABPM rather than HBPM. It has been reported that 40% of treated hypertensive patients with normal home BP using HBPM had high BP when monitored with 24-h ABPM [16]. Thus, some of our adequately controlled patients may actually have had masked uncontrolled hypertension. Last, the parameters used to assess lability were not able to reflect the full complexity of perioperative BP patterns.